Ubiquitin conjugating enzyme E2 L3 promoted tumor growth of NSCLC through accelerating p27kip1 ubiquitination and degradation
- PMID: 29137415
- PMCID: PMC5663587
- DOI: 10.18632/oncotarget.20449
Ubiquitin conjugating enzyme E2 L3 promoted tumor growth of NSCLC through accelerating p27kip1 ubiquitination and degradation
Abstract
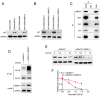
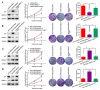
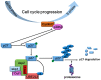
The molecular pathogenesis of human lung cancer has not been completely clarified. Here, we reported that UBE2L3, a member of the ubiquitin-conjugating enzymes (E2s), were overexpressed in non-small-cell lung cancer (NSCLC) tissues compared with the non-tumor tissues. High expression of UBE2L3 was correlated with advanced tumor stage and adverse outcomes. Knockdown of UBE2L3 inhibited NSCLC cell growth while ectopic expression of UBE2L3 promoted NSCLC cell growth in a cell cycle dependent manner. The results of subcutaneous tumor xenograft studies revealed that knockdown of UBE2L3 attenuated the in vivo tumor growth. Mechanistically, we observed that UBE2L3 could interact with F-box protein Skp2, a member of the SCF (Skp2) ubiquitin ligase complex, and thus promoted the ubiquitination and proteasomal degradation of p27kip1. Furthermore, NSCLC cases with high level of UBE2L3 and low level of p27kip1 had worst prognosis, suggesting that combination of UBE2L3 and p27kip1 is a more powerful prognostic marker for NSCLC patients. Taken together, the current study presented a novel marker for predicting prognosis and a potential therapeutic target for NSCLC patients.
Keywords: NSCLC; UBE2L3; degradation; p27kip1; ubiquitination.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures




Similar articles
-
The membrane-associated ubiquitin ligase MARCHF8 stabilizes the human papillomavirus oncoprotein E7 by degrading CUL1 and UBE2L3 in head and neck cancer.bioRxiv [Preprint]. 2023 Nov 4:2023.11.03.565564. doi: 10.1101/2023.11.03.565564. bioRxiv. 2023. Update in: J Virol. 2024 Feb 20;98(2):e0172623. doi: 10.1128/jvi.01726-23. PMID: 37961092 Free PMC article. Updated. Preprint.
-
Mechanism and Disease Association With a Ubiquitin Conjugating E2 Enzyme: UBE2L3.Front Immunol. 2022 Feb 21;13:793610. doi: 10.3389/fimmu.2022.793610. eCollection 2022. Front Immunol. 2022. PMID: 35265070 Free PMC article. Review.
-
SIRT2 inhibits non-small cell lung cancer cell growth through impairing Skp2-mediated p27 degradation.Oncotarget. 2016 Apr 5;7(14):18927-39. doi: 10.18632/oncotarget.7816. Oncotarget. 2016. PMID: 26942878 Free PMC article.
-
EGCG stabilizes p27kip1 in E2-stimulated MCF-7 cells through down-regulation of the Skp2 protein.Endocrinology. 2008 Dec;149(12):5972-83. doi: 10.1210/en.2008-0408. Epub 2008 Aug 21. Endocrinology. 2008. PMID: 18719023
-
Ubiquitin-conjugating enzyme E2C: a potential cancer biomarker.Int J Biochem Cell Biol. 2014 Feb;47:113-7. doi: 10.1016/j.biocel.2013.11.023. Epub 2013 Dec 17. Int J Biochem Cell Biol. 2014. PMID: 24361302 Review.
Cited by
-
Stabilization of p27Kip1/CDKN1B by UBCH7/UBE2L3 catalyzed ubiquitinylation: a new paradigm in cell-cycle control.FASEB J. 2019 Jan;33(1):1235-1247. doi: 10.1096/fj.201800960R. Epub 2018 Aug 16. FASEB J. 2019. PMID: 30113882 Free PMC article.
-
HPV-mediated nuclear export of HP1γ drives cervical tumorigenesis by downregulation of p53.Cell Death Differ. 2020 Sep;27(9):2537-2551. doi: 10.1038/s41418-020-0520-5. Epub 2020 Mar 23. Cell Death Differ. 2020. PMID: 32203172 Free PMC article.
-
TRIM27 acts as an oncogene and regulates cell proliferation and metastasis in non-small cell lung cancer through SIX3-β-catenin signaling.Aging (Albany NY). 2020 Dec 2;12(24):25564-25580. doi: 10.18632/aging.104163. Epub 2020 Dec 2. Aging (Albany NY). 2020. PMID: 33264103 Free PMC article.
-
The Molecular Basis of Ubiquitin-Conjugating Enzymes (E2s) as a Potential Target for Cancer Therapy.Int J Mol Sci. 2021 Mar 26;22(7):3440. doi: 10.3390/ijms22073440. Int J Mol Sci. 2021. PMID: 33810518 Free PMC article. Review.
-
UBE2L3 promotes lung adenocarcinoma invasion and metastasis through the GSK-3β/Snail signaling pathway.Am J Transl Res. 2022 Jul 15;14(7):4549-4561. eCollection 2022. Am J Transl Res. 2022. PMID: 35958458 Free PMC article.
References
-
- Heist RS, Engelman JA. SnapShot: non-small cell lung cancer. Cancer Cell. 2012;21:448.e2. https://doi.org/10.1016/j.ccr.2012.03.007. - DOI - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. https://doi.org/10.3322/caac.21166. - DOI - PubMed
-
- Liu Z, Zanata SM, Kim J, Peterson MA, Di Vizio D, Chirieac LR, Pyne S, Agostini M, Freeman MR, Loda M. The ubiquitin-specific protease USP2a prevents endocytosis-mediated EGFR degradation. Oncogene. 2013;32:1660–9. https://doi.org/10.1038/onc.2012.188. - DOI - PMC - PubMed
-
- De Rosa V, Iommelli F, Monti M, Fonti R, Votta G, Stoppelli MP, Del Vecchio S. Reversal of Warburg Effect and Reactivation of Oxidative Phosphorylation by Differential Inhibition of EGFR Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res. 2015;21:5110–20. https://doi.org/10.1158/1078-0432.ccr-15-0375. - DOI - PubMed
-
- Wrangle J, Machida EO, Danilova L, Hulbert A, Franco N, Zhang W, Glockner SC, Tessema M, Van Neste L, Easwaran H, Schuebel KE, Licchesi J, Hooker CM, et al. Functional identification of cancer-specific methylation of CDO1, HOXA9, and TAC1 for the diagnosis of lung cancer. Clin Cancer Res. 2014;20:1856–64. https://doi.org/10.1158/1078-0432.ccr-13-2109. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

