Human hyaluronic acid synthase-1 promotes malignant transformation via epithelial-to-mesenchymal transition, micronucleation and centrosome abnormalities
- PMID: 29137675
- PMCID: PMC5686803
- DOI: 10.1186/s12964-017-0204-z
Human hyaluronic acid synthase-1 promotes malignant transformation via epithelial-to-mesenchymal transition, micronucleation and centrosome abnormalities
Abstract
Background: Human hyaluronic acid (HA) molecules are synthesized by three membrane spanning Hyaluronic Acid Synthases (HAS1, HAS2 and HAS3). Of the three, HAS1 is found to be localized more into the cytoplasmic space where it synthesizes intracellular HA. HA is a ubiquitous glycosaminoglycan, mainly present in the extracellular matrix (ECM) and on the cell surface, but are also detected intracellularly. Accumulation of HA in cancer cells, the cancer-surrounding stroma, and ECM is generally considered an independent prognostic factors for patients. Higher HA production also correlates with higher tumor grade and more genetic heterogeneity in multiple cancer types which is known to contribute to drug resistance and results in treatment failure. Tumor heterogeneity and intra-tumor clonal diversity are major challenges for diagnosis and treatment. Identification of the driver pathway(s) that initiate genomic instability, tumor heterogeneity and subsequent phenotypic/clinical manifestations, are fundamental for the diagnosis and treatment of cancer. Thus far, no evidence was shown to correlate intracellular HA status (produced by HAS1) and the generation of genetic diversity in tumors.
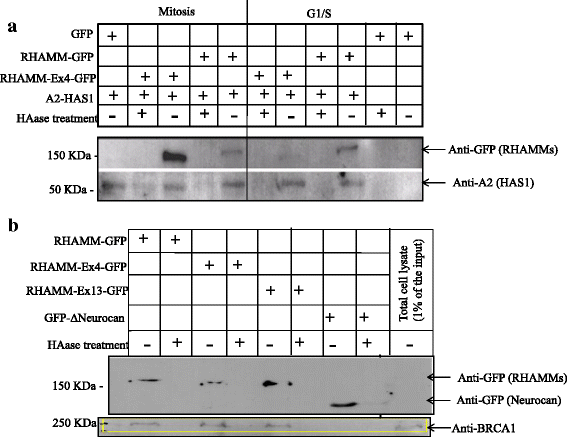
Methods: We tested different cell lines engineered to induce HAS1 expression. We measured the epithelial traits, centrosomal abnormalities, micronucleation and polynucleation of those HAS1-expressing cells. We performed real-time PCR, 3D cell culture assay, confocal microscopy, immunoblots and HA-capture methods.
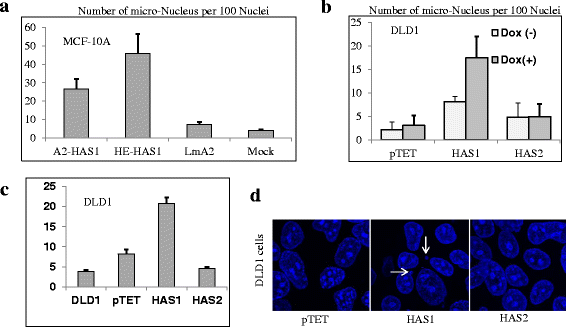
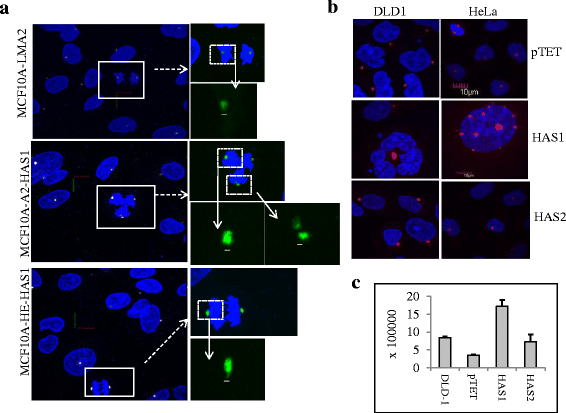
Results: Our results demonstrate that overexpression of HAS1 induces loss of epithelial traits, increases centrosomal abnormalities, micronucleation and polynucleation, which together indicate manifestation of malignant transformation, intratumoral genetic heterogeneity, and possibly create suitable niche for cancer stem cells generation.
Conclusions: The intracellular HA produced by HAS1 can aggravate genomic instability and intratumor heterogeneity, pointing to a fundamental role of intracellular HA in cancer initiation and progression.
Keywords: Chromosomal instability and Centrosome abnormalities; Epithelial-to-Mesenchymal transition; Genetic heterogeneity; Genomic instability; Hyaluronic acid Synthase-1; Malignant transformation; Micronucleus.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Auvinen P, Tammi R, Parkkinen J, Tammi M, Agren U, Johansson R, Hirvikoski P, Eskelinen M, Kosma VM. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol. 2000;156:529–536. doi: 10.1016/S0002-9440(10)64757-8. - DOI - PMC - PubMed
-
- Auvinen P, Rilla K, Tumelius R, Tammi M, Sironen R, Soini Y, Kosma VM, Mannermaa A, Viikari J, Tammi R. Hyaluronan synthases (HAS1-3) in stromal and malignant cells correlate with breast cancer grade and predict patient survival. Breast Cancer Res Treat. 2014;143:277–286. doi: 10.1007/s10549-013-2804-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

