Long-range stress transmission guides endothelial gap formation
- PMID: 29137986
- PMCID: PMC5761675
- DOI: 10.1016/j.bbrc.2017.11.066
Long-range stress transmission guides endothelial gap formation
Abstract
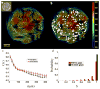
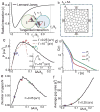
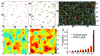
In endothelial gap formation, local tractions exerted by the cell upon its basal adhesions are thought to exceed balancing tensile stresses exerted across the cell-cell junction, thus causing the junction to rupture. To test this idea, we mapped evolving tractions, intercellular stresses, and corresponding growth of paracellular gaps in response to agonist challenge. Contrary to expectation, we found little to no relationship between local tensile stresses and gap formation. Instead, we discovered that intercellular stresses were aligned into striking multi-cellular domains punctuated by defects in stress alignment. Surprisingly, gaps emerged preferentially not at stress hotspots, as predicted, but rather at stress defects. This unexpected behavior is captured by a minimal model of the cell layer as a jammed assembly of cohesive particles undergoing plastic rearrangements under tension. Together, experiments and model suggest a new physical picture in which gap formation, and its consequent effect on endothelial permeability, is determined not by a local stress imbalance at a cell-cell junction but rather by emergence of non-local, cooperative stress reorganization across the cellular collective.
Keywords: Barrier function; Endothelium; Gaps; Stress; Traction.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

References
-
- Hotchkiss RS, Karl IE. The Pathophysiology and Treatment of Sepsis. New England Journal of Medicine. 2003;348:138–150. - PubMed
-
- Ware L, Mathay M. The Acute Respiratory Distress Syndrome. New England Journal of Medicine. 2000;342:1334–1349. - PubMed
-
- Mehta D, Bhattacharya J, Matthay MA, Malik AB. Integrated control of lung fluid balance. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2004;287:1081–1090. - PubMed
-
- Petit V, Thiery JP. Focal Adhesions: Structure and Dynamics. Biology of the Cell. 2012;92:477–494. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

