Defining failed induction of labor
- PMID: 29138035
- PMCID: PMC5819749
- DOI: 10.1016/j.ajog.2017.11.556
Defining failed induction of labor
Abstract
Background: While there are well-accepted standards for the diagnosis of arrested active-phase labor, the definition of a "failed" induction of labor remains less certain. One approach to diagnosing a failed induction is based on the duration of the latent phase. However, a standard for the minimum duration that the latent phase of a labor induction should continue, absent acute maternal or fetal indications for cesarean delivery, remains lacking.
Objective: The objective of this study was to determine the frequency of adverse maternal and perinatal outcomes as a function of the duration of the latent phase among nulliparous women undergoing labor induction.
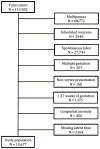
Study design: This study is based on data from an obstetric cohort of women delivering at 25 US hospitals from 2008 through 2011. Nulliparous women who had a term singleton gestation in the cephalic presentation were eligible for this analysis if they underwent a labor induction. Consistent with prior studies, the latent phase was determined to begin once cervical ripening had ended, oxytocin was initiated, and rupture of membranes had occurred, and was determined to end once 5-cm dilation was achieved. The frequencies of cesarean delivery, as well as of adverse maternal (eg, postpartum hemorrhage, chorioamnionitis) and perinatal (eg, a composite frequency of seizures, sepsis, bone or nerve injury, encephalopathy, or death) outcomes, were compared as a function of the duration of the latent phase (analyzed with time both as a continuous measure and categorized in 3-hour increments).
Results: A total of 10,677 women were available for analysis. In the vast majority (96.4%) of women, the active phase had been reached by 15 hours. The longer the duration of a woman's latent phase, the greater her chance of ultimately undergoing a cesarean delivery (P < .001, for time both as a continuous and categorical independent variable), although >40% of women whose latent phase lasted ≥18 hours still had a vaginal delivery. Several maternal morbidities, such as postpartum hemorrhage (P < .001) and chorioamnionitis (P < .001), increased in frequency as the length of latent phase increased. Conversely, the frequencies of most adverse perinatal outcomes were statistically stable over time.
Conclusion: The large majority of women undergoing labor induction will have entered the active phase by 15 hours after oxytocin has started and rupture of membranes has occurred. Maternal adverse outcomes become statistically more frequent with greater time in the latent phase, although the absolute increase in frequency is relatively small. These data suggest that cesarean delivery should not be undertaken during the latent phase prior to at least 15 hours after oxytocin and rupture of membranes have occurred. The decision to continue labor beyond this point should be individualized, and may take into account factors such as other evidence of labor progress.
Keywords: labor induction; latent phase; outcomes.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
No author has a conflict of interest
Figures
References
-
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1–65. - PubMed
-
- Rouse DJ, Owen J, Hauth JC. Criteria for failed labor induction: prospective evaluation of a standardized protocol. Obstet Gynecol. 2000;96:671–7. - PubMed
-
- Simon CE, Grobman WA. When has an induction failed? Obstet Gynecol. 2005;105:705–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 HD040500/HD/NICHD NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- U10 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- UG1 HD053097/HD/NICHD NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- U10 HD040485/HD/NICHD NIH HHS/United States
- U10 HD034116/HD/NICHD NIH HHS/United States
- U10 HD027869/HD/NICHD NIH HHS/United States
- U10 HD027917/HD/NICHD NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- U10 HD040560/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- U10 HD040512/HD/NICHD NIH HHS/United States
- U10 HD040545/HD/NICHD NIH HHS/United States
- U01 HD036801/HD/NICHD NIH HHS/United States
- U10 HD053118/HD/NICHD NIH HHS/United States
- UL1 RR024989/RR/NCRR NIH HHS/United States
- UL1 RR025764/RR/NCRR NIH HHS/United States
- UG1 HD034116/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
- U10 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- U10 HD053097/HD/NICHD NIH HHS/United States
- U10 HD021410/HD/NICHD NIH HHS/United States
- U10 HD036801/HD/NICHD NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical