Thrombospondin-4 in tissue remodeling
- PMID: 29138119
- PMCID: PMC6005712
- DOI: 10.1016/j.matbio.2017.11.006
Thrombospondin-4 in tissue remodeling
Abstract
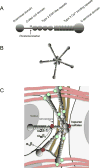
Thrombospondin-4 (TSP-4) belongs to the thrombospondin protein family that consists of five highly homologous members. A number of novel functions have been recently assigned to TSP-4 in cardiovascular and nervous systems, inflammation, cancer, and the motor unit, which have attracted attention to this extracellular matrix (ECM) protein. These newly discovered functions set TSP-4 apart from other thrombospondins. For example, TSP-4 promotes angiogenesis while other TSPs either prevent it or have no effect on new blood vessel growth; TSP-4 reduces fibrosis and collagen production while TSP-1 and TSP-2 promote fibrosis in several organs; unlike other TSPs, TSP-4 appears to have some structural functions in ECM. The current information about TSP-4 functions in different organs and physiological systems suggests that this evolutionary conserved protein is a major regulator of the extracellular matrix (ECM) organization and production and tissue remodeling during the embryonic development and response to injury. In this review article, we summarize the properties and functions of TSP-4 and discuss its role in tissue remodeling.
Keywords: Angiogenesis; Extracellular matrix (ECM); Fibrosis; Myocardium; Nociception; Skeletal muscle.
Copyright © 2017. Published by Elsevier B.V.
Figures
References
-
- Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007;12:331–343. - PubMed
-
- Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. - PubMed
-
- Stenina OI, Topol EJ, Plow EF. Thrombospondins, their polymorphisms, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2007;27:1886–1894. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

