Low Neonatal Plasma n-6/n-3 PUFA Ratios Regulate Offspring Adipogenic Potential and Condition Adult Obesity Resistance
- PMID: 29138256
- PMCID: PMC5860857
- DOI: 10.2337/db17-0890
Low Neonatal Plasma n-6/n-3 PUFA Ratios Regulate Offspring Adipogenic Potential and Condition Adult Obesity Resistance
Abstract
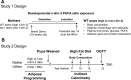
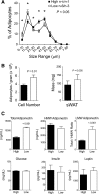
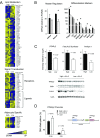
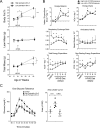
Adipose tissue expansion progresses rapidly during postnatal life, influenced by both prenatal maternal factors and postnatal developmental cues. The ratio of omega-6 (n-6) relative to n-3 polyunsaturated fatty acids (PUFAs) is believed to regulate perinatal adipogenesis, but the cellular mechanisms and long-term effects are not well understood. We lowered the fetal and postnatal n-6/n-3 PUFA ratio exposure in wild-type offspring under standard maternal dietary fat amounts to test the effects of low n-6/n-3 ratios on offspring adipogenesis and adipogenic potential. Relative to wild-type pups receiving high perinatal n-6/n-3 ratios, subcutaneous adipose tissue in 14-day-old wild-type pups receiving low n-6/n-3 ratios had more adipocytes that were smaller in size; decreased Pparγ2, Fabp4, and Plin1; several lipid metabolism mRNAs; coincident hypermethylation of the PPARγ2 proximal promoter; and elevated circulating adiponectin. As adults, offspring that received low perinatal n-6/n-3 ratios were diet-induced obesity (DIO) resistant and had a lower positive energy balance and energy intake, greater lipid fuel preference and non-resting energy expenditure, one-half the body fat, and better glucose clearance. Together, the findings support a model in which low early-life n-6/n-3 ratios remodel adipose morphology to increase circulating adiponectin, resulting in a persistent adult phenotype with improved metabolic flexibility that prevents DIO.
© 2018 by the American Diabetes Association.
Figures

Comment in
-
A Weighty Matter: Can PUFAs in Pregnancy Prevent Obesity?Diabetes. 2018 Apr;67(4):548-549. doi: 10.2337/dbi17-0051. Diabetes. 2018. PMID: 29559511 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

