Coupling shRNA screens with single-cell RNA-seq identifies a dual role for mTOR in reprogramming-induced senescence
- PMID: 29138277
- PMCID: PMC5733499
- DOI: 10.1101/gad.297796.117
Coupling shRNA screens with single-cell RNA-seq identifies a dual role for mTOR in reprogramming-induced senescence
Abstract
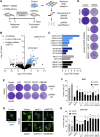
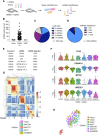
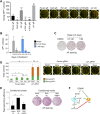
Expression of the transcription factors OCT4, SOX2, KLF4, and cMYC (OSKM) reprograms somatic cells into induced pluripotent stem cells (iPSCs). Reprogramming is a slow and inefficient process, suggesting the presence of safeguarding mechanisms that counteract cell fate conversion. One such mechanism is senescence. To identify modulators of reprogramming-induced senescence, we performed a genome-wide shRNA screen in primary human fibroblasts expressing OSKM. In the screen, we identified novel mediators of OSKM-induced senescence and validated previously implicated genes such as CDKN1A We developed an innovative approach that integrates single-cell RNA sequencing (scRNA-seq) with the shRNA screen to investigate the mechanism of action of the identified candidates. Our data unveiled regulation of senescence as a novel way by which mechanistic target of rapamycin (mTOR) influences reprogramming. On one hand, mTOR inhibition blunts the induction of cyclin-dependent kinase (CDK) inhibitors (CDKIs), including p16INK4a, p21CIP1, and p15INK4b, preventing OSKM-induced senescence. On the other hand, inhibition of mTOR blunts the senescence-associated secretory phenotype (SASP), which itself favors reprogramming. These contrasting actions contribute to explain the complex effect that mTOR has on reprogramming. Overall, our study highlights the advantage of combining functional screens with scRNA-seq to accelerate the discovery of pathways controlling complex phenotypes.
Keywords: SASP; iPSCs; senescence; shRNA screens; single-cell RNA-seq.
© 2017 Aarts et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
-
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. 2008. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133: 1006–1018. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
