The genetics of PKMζ and memory maintenance
- PMID: 29138296
- PMCID: PMC6171341
- DOI: 10.1126/scisignal.aao2327
The genetics of PKMζ and memory maintenance
Abstract
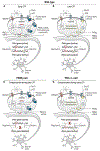
Elucidating the molecular mechanisms that maintain long-term memory is a fundamental goal of neuroscience. Accumulating evidence suggests that persistent signaling by the atypical protein kinase C (PKC) isoform protein kinase Mζ (PKMζ) might maintain synaptic long-term potentiation (LTP) and long-term memory. However, the role of PKMζ has been challenged by genetic data from PKMζ-knockout mice showing intact LTP and long-term memory. Moreover, the PKMζ inhibitor peptide ζ inhibitory peptide (ZIP) reverses LTP and erases memory in both wild-type and knockout mice. Data from four papers using additional isoform-specific genetic approaches have helped to reconcile these conflicting findings. First, a PKMζ-antisense approach showed that LTP and long-term memory in PKMζ-knockout mice are mediated through a compensatory mechanism that depends on another ZIP-sensitive atypical isoform, PKCι/λ. Second, short hairpin RNAs decreasing the amounts of individual atypical isoforms without inducing compensation disrupted memory in different temporal phases. PKCι/λ knockdown disrupted short-term memory, whereas PKMζ knockdown specifically erased long-term memory. Third, conditional PKCι/λ knockout induced compensation by rapidly activating PKMζ to preserve short-term memory. Fourth, a dominant-negative approach in the model system Aplysia revealed that multiple PKCs form PKMs to sustain different types of long-term synaptic facilitation, with atypical PKM maintaining synaptic plasticity similar to LTP. Thus, under physiological conditions, PKMζ is the principal PKC isoform that maintains LTP and long-term memory. PKCι/λ can compensate for PKMζ, and because other isoforms could also maintain synaptic facilitation, there may be a hierarchy of compensatory mechanisms maintaining memory if PKMζ malfunctions.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
References
-
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF, Learning induces long-term potentiation in the hippocampus. Science 313, 1093–1097 (2006). - PubMed
-
- Morris RGM, NMDA receptors and memory encoding. Neuropharmacology 74, 32–40 (2013). - PubMed
-
- Crick F, Memory and molecular turnover. Nature 312, 101 (1984). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

