Sparsity enables estimation of both subcortical and cortical activity from MEG and EEG
- PMID: 29138310
- PMCID: PMC5715738
- DOI: 10.1073/pnas.1705414114
Sparsity enables estimation of both subcortical and cortical activity from MEG and EEG
Abstract
Subcortical structures play a critical role in brain function. However, options for assessing electrophysiological activity in these structures are limited. Electromagnetic fields generated by neuronal activity in subcortical structures can be recorded noninvasively, using magnetoencephalography (MEG) and electroencephalography (EEG). However, these subcortical signals are much weaker than those generated by cortical activity. In addition, we show here that it is difficult to resolve subcortical sources because distributed cortical activity can explain the MEG and EEG patterns generated by deep sources. We then demonstrate that if the cortical activity is spatially sparse, both cortical and subcortical sources can be resolved with M/EEG. Building on this insight, we develop a hierarchical sparse inverse solution for M/EEG. We assess the performance of this algorithm on realistic simulations and auditory evoked response data, and show that thalamic and brainstem sources can be correctly estimated in the presence of cortical activity. Our work provides alternative perspectives and tools for characterizing electrophysiological activity in subcortical structures in the human brain.
Keywords: EEG; MEG; source localization; sparsity; subcortical structures.
Copyright © 2017 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



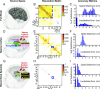
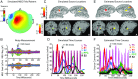

References
-
- Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. - PubMed
-
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. - PubMed
-
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2016;33:325–340. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

