Dual function for Tango1 in secretion of bulky cargo and in ER-Golgi morphology
- PMID: 29138315
- PMCID: PMC5715762
- DOI: 10.1073/pnas.1711408114
Dual function for Tango1 in secretion of bulky cargo and in ER-Golgi morphology
Abstract
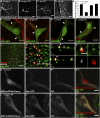
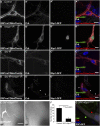
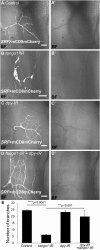
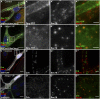
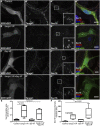
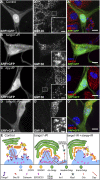
Tango1 enables ER-to-Golgi trafficking of large proteins. We show here that loss of Tango1, in addition to disrupting protein secretion and ER/Golgi morphology, causes ER stress and defects in cell shape. We find that the previously observed dependence of smaller cargos on Tango1 is a secondary effect. If large cargos like Dumpy, which we identify as a Tango1 cargo, are removed from the cell, nonbulky proteins reenter the secretory pathway. Removal of blocking cargo also restores cell morphology and attenuates the ER-stress response. Thus, failures in the secretion of nonbulky proteins, ER stress, and defective cell morphology are secondary consequences of bulky cargo retention. By contrast, ER/Golgi defects in Tango1-depleted cells persist in the absence of bulky cargo, showing that they are due to a secretion-independent function of Tango1. Therefore, maintenance of ER/Golgi architecture and bulky cargo transport are the primary functions for Tango1.
Keywords: ER stress; ERES; ERGIC; GM130; Sec16.
Copyright © 2017 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Saito K, et al. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009;136:891–902. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

