A Phase I Clinical Trial of the Poly(ADP-ribose) Polymerase Inhibitor Veliparib and Weekly Topotecan in Patients with Solid Tumors
- PMID: 29138343
- PMCID: PMC7580251
- DOI: 10.1158/1078-0432.CCR-17-1590
A Phase I Clinical Trial of the Poly(ADP-ribose) Polymerase Inhibitor Veliparib and Weekly Topotecan in Patients with Solid Tumors
Abstract
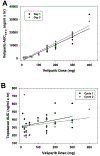
Purpose: To determine the dose limiting toxicities (DLT), maximum tolerated dose (MTD), and recommended phase II dose (RP2D) of veliparib in combination with weekly topotecan in patients with solid tumors. Correlative studies were included to assess the impact of topotecan and veliparib on poly(ADP-ribose) levels in peripheral blood mononuclear cells, serum pharmacokinetics of both agents, and potential association of germline repair gene mutations with outcome.Experimental Design: Eligible patients had metastatic nonhematologic malignancies with measurable disease. Using a 3 + 3 design, patients were treated with veliparib orally twice daily on days 1-3, 8-10, and 15-17 and topotecan intravenously on days 2, 9, and 16 every 28 days. Tumor responses were assessed by RECIST.Results: Of 58 patients enrolled, 51 were evaluable for the primary endpoint. The MTD and RP2D was veliparib 300 mg twice daily on days 1-3, 8-10, and 15-17 along with topotecan 3 mg/m2 on days 2, 9, and 16 of a 28-day cycle. DLTs were grade 4 neutropenia lasting >5 days. The median number of cycles was 2 (1-26). The objective response rate was 10%, with 1 complete and 4 partial responses. Twenty-two patients (42%) had stable disease ranging from 4 to 26 cycles. Patients with germline BRCA1, BRCA2, or RAD51D mutations remained on study longer than those without homologous recombination repair (HRR) gene mutations (median 4 vs. 2 cycles).Conclusions: Weekly topotecan in combination with veliparib has a manageable safety profile and appears to warrant further investigation. Clin Cancer Res; 24(4); 744-52. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures
References
-
- Khadka DB, Cho WJ. Topoisomerase inhibitors as anticancer agents: a patent update. Expert Opin Ther Pat 2013;23(8):1033–56. - PubMed
-
- Kumler I, Brunner N, Stenvang J, Balslev E, Nielsen DL. A systematic review on topoisomerase 1 inhibition in the treatment of metastatic breast cancer. Breast Cancer Res Treat 2013;138(2):347–58. - PubMed
-
- Beretta GL, Gatti L, Perego P, Zaffaroni N. Camptothecin resistance in cancer: insights into the molecular mechanisms of a DNA-damaging drug. Curr Med Chem 2013;20(12):1541–65. - PubMed
-
- Moukharskaya J, Verschraegen C. Topoisomerase 1 inhibitors and cancer therapy. Hematol Oncol Clin North Am 2012;26(3):507–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

