The photocycle of orange carotenoid protein conceals distinct intermediates and asynchronous changes in the carotenoid and protein components
- PMID: 29138423
- PMCID: PMC5686206
- DOI: 10.1038/s41598-017-15520-4
The photocycle of orange carotenoid protein conceals distinct intermediates and asynchronous changes in the carotenoid and protein components
Abstract
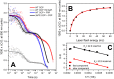
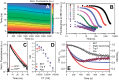
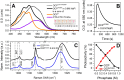
The 35-kDa Orange Carotenoid Protein (OCP) is responsible for photoprotection in cyanobacteria. It acts as a light intensity sensor and efficient quencher of phycobilisome excitation. Photoactivation triggers large-scale conformational rearrangements to convert OCP from the orange OCPO state to the red active signaling state, OCPR, as demonstrated by various structural methods. Such rearrangements imply a complete, yet reversible separation of structural domains and translocation of the carotenoid. Recently, dynamic crystallography of OCPO suggested the existence of photocycle intermediates with small-scale rearrangements that may trigger further transitions. In this study, we took advantage of single 7 ns laser pulses to study carotenoid absorption transients in OCP on the time-scale from 100 ns to 10 s, which allowed us to detect a red intermediate state preceding the red signaling state, OCPR. In addition, time-resolved fluorescence spectroscopy and the assignment of carotenoid-induced quenching of different tryptophan residues derived thereof revealed a novel orange intermediate state, which appears during the relaxation of photoactivated OCPR to OCPO. Our results show asynchronous changes between the carotenoid- and protein-associated kinetic components in a refined mechanistic model of the OCP photocycle, but also introduce new kinetic signatures for future studies of OCP photoactivity and photoprotection.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

