Modelling the effect of beliefs about asthma medication and treatment intrusiveness on adherence and preference for once-daily vs. twice-daily medication
- PMID: 29138431
- PMCID: PMC5686129
- DOI: 10.1038/s41533-017-0061-7
Modelling the effect of beliefs about asthma medication and treatment intrusiveness on adherence and preference for once-daily vs. twice-daily medication
Abstract
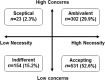
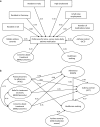
People with asthma who do not adhere to their maintenance medication may experience poorer asthma control and need more healthcare support than those who adhere. People (N = 1010) aged 18-55 years with self-reported asthma, taking one or more asthma maintenance medication(s), from five European countries, participated in a survey using validated scales (Medication Adherence Report Scale [MARS], Asthma Control Test™ [ACT], Beliefs about Medicine Questionnaire [BMQ] and the Asthma Treatment Intrusiveness Questionnaire [ATIQ]). We performed a post hoc evaluation of adherence to maintenance medication, asthma control, beliefs about medication, preferences for once-daily vs. twice-daily asthma maintenance medication and treatment intrusiveness, using structural equation modelling to investigate the relationships between these factors. Most participants reported potential problems with asthma control (ACT < 19: 76.8% [n = 776]), low adherence (median MARS = 3.40) and preferred once-daily medication (73.5% [n = 742/1010]). Non-adherence was associated with worse asthma control (r = 0.262 [P < 0.001]) and a expressed preference for once-daily medication over a "twice daily medication that works slightly better" (test statistic [T] = 2.970 [P = 0.003]). Participants reporting non-adherence/preferring once-daily medication had negative beliefs about their treatment (BMQ necessity-concerns differential: r = 0.437 [P < 0.001]/T = 6.886 [P < 0.001]) and found medication intrusive (ATIQ: r = -0.422 [P < 0.001]/T = 2.689[P = 0.007]). Structural equation modelling showed complex relationships between variables, including: (1) high concerns about treatment associated with increased perceived treatment intrusiveness and reduced adherence, which influenced asthma control; (2) high concerns about treatment and healthcare seeking behaviour, which were predictive of preferring twice-daily asthma medication. Concerns about medication and perceived treatment intrusiveness were predictive of poor adherence, and were associated with preference for once-daily asthma medication. Confirm the utility of the PAPA model and NCF in explaining nonadherence linked to poor asthma control.
Conflict of interest statement
S.C. has undertaken consultancy work for University College London spin-out company Spoonful of Sugar, including work commissioned by GlaxoSmithKline. H.S. is a GlaxoSmithKline employee and holds GlaxoSmithKline stocks. P.D. and G.S. are former GlaxoSmithKline employees and hold GlaxoSmithKline stocks. N.V. is an employee of Healthcare Research Worldwide, commissioned by GlaxoSmithKline to conduct the original market research survey. D.P. declares the following conflicts of interest: board membership with Aerocrine, Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis and Teva Pharmaceuticals; consultancy with Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer and Teva Pharmaceuticals; grants and unrestricted funding for investigator-initiated studies (conducted through Research in Real-Life Ltd and Observational and Pragmatic Research Institute Pte Ltd) from the UK National Health Service, British Lung Foundation, Aerocrine, AKL Ltd, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Eli Lilly, GlaxoSmithKline, Meda, Merck, Mundipharma, Napp, Novartis, Orion, Pfizer, Respiratory Effectiveness Group, Takeda, Teva Pharmaceuticals and Zentiva; payments for lectures/speaking from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, Skyepharma, Takeda and Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma and Teva Pharmaceuticals; patents (planned, pending or issued) from AKL Ltd; payment for the development of educational materials from GlaxoSmithKline and Novartis; stock/stock options from AKL Ltd, which produces phytopharmaceuticals; ownership of 80% of Research in Real Life Ltd, 75% of the social enterprise Optimum Patient Care Ltd and 75% of Observational and Pragmatic Research Institute Pte Ltd; payment for travel/accommodations/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis and Teva Pharmaceuticals; funding for patient enrolment or completion of research from Almirral, Chiesi, Teva Pharmaceuticals and Zentiva; and peer review for grant committees of the Medical Research Council (2014), Efficacy and Mechanism Evaluation programme (2012), HTA (2014). R.H. is a member of the Medical Innovation Academic Consortium, the academic arm of CASMI. R.H has undertaken speaker engagements with honoraria from the following organisations AbbVie, Amgen, Biogen, Idec, Gilead Sciences, GlaxoSmithKline, Janssen, Pfizer, Roche, Shire Pharmaceuticals, MSD, Astellas, AstraZeneca, DRSU, Novartis, Universitatsklinikum Hamburg-Eppendorf, Teva Pharmaceuticals. Professor Horne was supported by Asthma UK Centre for Applied Research (AUKCAR) and the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) North Thames at Bart's Health NHS Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Figures
References
-
- Akinbami LJ, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012;94:1–8. - PubMed
-
- Global Initiative for Asthma (GINA) The Global Burden of Asthmahttp://ginasthma.org/gina-reports/ (2017).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

