Development of novel ultrashort antimicrobial peptide nanoparticles with potent antimicrobial and antibiofilm activities against multidrug-resistant bacteria
- PMID: 29138537
- PMCID: PMC5679673
- DOI: 10.2147/DDDT.S147450
Development of novel ultrashort antimicrobial peptide nanoparticles with potent antimicrobial and antibiofilm activities against multidrug-resistant bacteria
Abstract
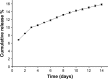
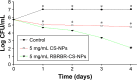
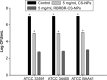
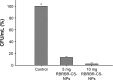
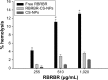
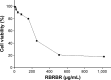
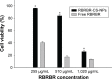
Conventional antibiotics are facing strong microbial resistance that has recently reached critical levels. This situation is leading to significantly reduced therapeutic potential of a huge proportion of antimicrobial agents currently used in clinical settings. Antimicrobial peptides (AMPs) could provide the medical community with an alternative strategy to traditional antibiotics for combating microbial resistance. However, the development of AMPs into clinically useful antibiotics is hampered by their relatively low stability, toxicity, and high manufacturing costs. In this study, a novel in-house-designed potent ultrashort AMP named RBRBR was encapsulated into chitosan-based nanoparticles (CS-NPs) based on the ionotropic gelation method. The encapsulation efficacy reported for RBRBR into CS-NPs was 51.33%, with a loading capacity of 10.17%. The release kinetics of RBRBR from the nanocarrier exhibited slow release followed by progressive linear release for 14 days. The antibacterial kinetics of RBRBR-CS-NPs was tested against four strains of Staphylococcus aureus for 4 days, and the developed RBRBR-CS-NPs exhibited a 3-log decrease in the number of colonies when compared to CS-NP and a 5-log decrease when compared to control bacteria. The encapsulated peptide NP formulation managed to limit the toxicity of the free peptide against both mammalian cells and human erythrocytes. Additionally, the peptide NPs demonstrated up to 98% inhibition of biofilm formation when tested against biofilm-forming bacteria. Loading RBRBR into CS-NPs could represent an innovative approach to develop delivery systems based on NP technology for achieving potent antimicrobial effects against multidrug-resistant and biofilm-forming bacteria, with negligible systemic toxicity and reduced synthetic costs, thereby overcoming the obstructions to clinical development of AMPs.
Keywords: antibiofilm; drug delivery; nanoparticles; ultrashort antimicrobial peptides.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

