Efficacy of tiotropium and indacaterol monotherapy and their combination on dynamic lung hyperinflation in COPD: a random open-label crossover study
- PMID: 29138547
- PMCID: PMC5679691
- DOI: 10.2147/COPD.S149054
Efficacy of tiotropium and indacaterol monotherapy and their combination on dynamic lung hyperinflation in COPD: a random open-label crossover study
Abstract
Background and objective: The difference in efficacy of long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs) for dynamic lung hyperinflation (DLH) in COPD is unclear. The purpose of this study was to elucidate the difference in efficacy of LAMA and LABA alone and the combination thereof for DLH.
Subjects and methods: Thirty stable patients were enrolled and randomly divided into two groups following baseline measurements. One group was treated with 5 μg tiotropium (Respimat inhaler) for 4 weeks following a 4-week treatment with 150 μg indacaterol, while the other group was treated with indacaterol for 4 weeks following a 4-week treatment with tiotropium. For both groups, these treatments were followed by a combination of the two drugs for 4 weeks. Pulmonary function tests, including DLH evaluated by metronome-paced incremental hyperventilation and exercise tolerance evaluated by the shuttle-walk test, were performed at the end of each treatment period.
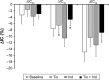
Results: In total, 23 patients completed this study. Both tiotropium and indacaterol alone significantly increased forced expiratory volume in 1 second, exercise tolerance, and improved health status. Tiotropium significantly improved DLH, but indacaterol did not. The combination therapy resulted in further improvements in lung function and exercise tolerance, but not in DLH.
Conclusion: The efficacy of tiotropium in inhibiting DLH following metronome-paced incremental hyperventilation may be superior to that of 150 μg indacaterol, although the effects on airflow obstruction were the same, and the combination therapy showed further improvement in airflow obstruction, but not in DLH.
Keywords: COPD; bronchodilator agents; clinical respiratory medicine; quality of life; respiratory function tests.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Efficacy of tiotropium and olodaterol combination therapy on dynamic lung hyperinflation evaluated by hyperventilation in COPD: an open-label, comparative before and after treatment study.Int J Chron Obstruct Pulmon Dis. 2019 May 27;14:1167-1176. doi: 10.2147/COPD.S201106. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 31213796 Free PMC article. Clinical Trial.
-
Efficacy and safety of indacaterol/glycopyrronium fixed-dose combination in mild-to-moderate COPD patients symptomatic on tiotropium in Korea: study protocol for a randomized controlled trial.Trials. 2017 Feb 22;18(1):80. doi: 10.1186/s13063-017-1800-3. Trials. 2017. PMID: 28228162 Free PMC article. Clinical Trial.
-
The impact of indacaterol/glycopyrronium fixed-dose combination versus tiotropium monotherapy on lung function and treatment preference: a randomized crossover study - the FAVOR study.Int J Chron Obstruct Pulmon Dis. 2017 Dec 22;13:69-77. doi: 10.2147/COPD.S146189. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 29317812 Free PMC article. Clinical Trial.
-
Efficacy of tiotropium-olodaterol fixed-dose combination in COPD.Int J Chron Obstruct Pulmon Dis. 2016 Dec 13;11:3163-3177. doi: 10.2147/COPD.S92840. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 28008243 Free PMC article. Review.
-
Clinical benefit of fixed-dose dual bronchodilation with glycopyrronium and indacaterol once daily in patients with chronic obstructive pulmonary disease: a systematic review.Int J Chron Obstruct Pulmon Dis. 2014 Apr 1;9:331-8. doi: 10.2147/COPD.S60362. eCollection 2014. Int J Chron Obstruct Pulmon Dis. 2014. PMID: 24729699 Free PMC article.
Cited by
-
Usefulness of a Newly Developed Spirometer to Measure Dynamic Lung Hyperinflation following Incremental Hyperventilation in Patients with Chronic Obstructive Pulmonary Disease.Intern Med. 2019 Jan 1;58(1):39-46. doi: 10.2169/internalmedicine.1212-18. Epub 2018 Aug 10. Intern Med. 2019. PMID: 30101930 Free PMC article.
-
Therapeutic Response to Single-Inhaler Triple Therapies in Moderate-to-Severe COPD.Respir Care. 2023 Mar;68(3):330-337. doi: 10.4187/respcare.10188. Respir Care. 2023. PMID: 36828578 Free PMC article.
-
Is the Metronome-Paced Tachypnea Test (MPT) Ready for Clinical Use? Accuracy of the MPT in a Prospective and Clinical Study.Respiration. 2019;97(6):569-575. doi: 10.1159/000496290. Epub 2019 Mar 14. Respiration. 2019. PMID: 30870858 Free PMC article.
-
Metronome-Paced Incremental Hyperventilation May Predict Exercise Tolerance and Dyspnea as a Surrogate for Dynamic Lung Hyperinflation During Exercise.Int J Chron Obstruct Pulmon Dis. 2020 May 15;15:1061-1069. doi: 10.2147/COPD.S246850. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 32523336 Free PMC article.
-
Longitudinal change of FEV1 and inspiratory capacity: clinical implication and relevance to exacerbation risk in patients with COPD.Int J Chron Obstruct Pulmon Dis. 2019 Feb 4;14:361-369. doi: 10.2147/COPD.S189384. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 30787605 Free PMC article.
References
-
- O’Donnell DE, Bertley JC, Chau LK, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med. 1997;155:109–115. - PubMed
-
- O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:770–777. - PubMed
-
- Belman MJ, Botnick WC, Shin JW. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153:967–975. - PubMed
-
- Battram C, Charlton SJ, Cuenoud B, et al. In vitro and in vivo pharmacological characterization of 5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-hydroxy-1H-quinolin-2-one (indacaterol), a novel inhaled β2 adrenoceptor agonist with a 24-h duration of action. J Pharmacol Exp Ther. 2006;317:762–770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

