Photoactivatable RNAi for cancer gene therapy triggered by near-infrared-irradiated single-walled carbon nanotubes
- PMID: 29138556
- PMCID: PMC5666115
- DOI: 10.2147/IJN.S141882
Photoactivatable RNAi for cancer gene therapy triggered by near-infrared-irradiated single-walled carbon nanotubes
Abstract
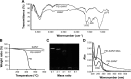
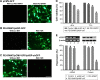
The efficacy of RNA interference (RNAi)-based cancer gene therapy is limited by its unexpected side effects, thus necessitating a strategy to precisely trigger conditional gene knockdown. In this study, we engineered a novel photoactivatable RNAi system, named as polyetherimide-modified single-wall carbon nanotube (PEI-SWNT)/pHSP-shT, that enables optogenetic control of targeted gene suppression in tumor cells. PEI-SWNT/pHSP-shT comprises a stimulus-responsive nanocarrier (PEI-SWNT), and an Hsp70B'-promoter-driven RNAi vector (pHSP-shT). In response to near-infrared (NIR) light irradiation, heating of PEI-SWNT in breast MCF-7 cells triggered gene knockdown targeting human telomerase reverse transcriptase through RNAi, with the gene-knockdown activity capable of being switched off by extinguishing the NIR. Furthermore, we demonstrated that the photoactivatable RNAi system exhibited higher antitumor activity by combining gene therapy and photothermal therapy, both in vitro and in vivo. Optogenetic control of RNAi based on an NIR-activated nanocarrier will potentially facilitate improved understanding of molecular-targeted gene therapy in human malignant tumors.
Keywords: Hsp70B′ promoter; RNAi; SWNT; near-infrared light response.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures





References
-
- Conde J, Ambrosome A, Hernandez Y, et al. 15 years on siRNA delivery: beyond the State-of-the-Art on inorganic nanoparticles for RNAi therapeutics. Nano Today. 2015;10(4):421–450.
-
- Kim H, Chung K, Lee S, Kim DH, Lee H. Near-infrared light-responsive nanomaterials for cancer theranostics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8(1):23–45. - PubMed
-
- Li D, Ma Y, Du J, et al. Tumor acidity/NIR controlled interaction of transformable nanoparticle with biological systems for cancer therapy. Nano Lett. 2017;17(5):2871–2878. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

