Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia
- PMID: 29138559
- PMCID: PMC5667789
- DOI: 10.2147/IJN.S145096
Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia
Abstract
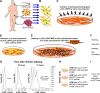
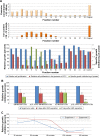
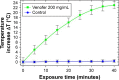
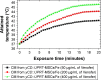
Magnetic hyperthermia, or the heating of tissues using magnetic materials, is a promising approach for treating cancer. We found that human mesenchymal stem cells (MSCs) isolated from various tissues and MSCs expressing the yeast cytosine deaminase∷uracil phosphoribosyl transferase suicide fusion gene (yCD∷UPRT) can be labeled with Venofer, an iron oxide carbohydrate nanoparticle. Venofer labeling did not affect cell proliferation or the ability to home to tumors. All Venofer-labeled MSCs released exosomes that contained iron oxide. Furthermore, these exosomes were efficiently endocytosed by tumor cells. Exosomes from Venofer-labeled MSCs expressing the yCD∷UPRT gene in the presence of the prodrug 5-fluorocytosine inhibited tumor growth in a dose-dependent fashion. The treated tumor cells were also effectively ablated following induction of hyperthermia using an external alternating magnetic field. Cumulatively, we found that magnetic nanoparticles packaged into MSC exosomes are efficiently endocytosed by tumor cells, facilitating targeted tumor cell ablation via magnetically induced hyperthermia.
Keywords: Venofer; iron oxide labeling; magnetic hyperthermia; mesenchymal stem cells; yCD∷UPRT-MSCs/Fe exosomes; yCD∷UPRT-exosomes.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



References
-
- Sawdon A, Weydemeyer E, Peng CA. Antitumor therapy using nanomaterial-mediated thermolysis. J Biomed Nanotechnol. 2014;10(9):1894–1917. - PubMed
-
- Altaner C. Stem Cell-Mediated Prodrug Gene Therapy of High-Grade Brain Tumors. In: Shah K, editor. Stem Cell Therapeutics for Cancer. New Jersey, USA: Wiley-Blackwell; 2013. pp. 57–72.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

