Efficacy of intravenous tigecycline in patients with Acinetobacter complex infections: results from 14 Phase III and Phase IV clinical trials
- PMID: 29138583
- PMCID: PMC5679678
- DOI: 10.2147/IDR.S143306
Efficacy of intravenous tigecycline in patients with Acinetobacter complex infections: results from 14 Phase III and Phase IV clinical trials
Abstract
Background: Acinetobacter infections, especially multidrug-resistant (MDR) Acinetobacter infections, are a global health problem. This study aimed to describe clinical outcomes in patients with confirmed Acinetobacter spp. isolates who were treated with tigecycline in randomized clinical trials.
Materials and methods: Data from 14 multinational, randomized (open-label or double-blind), and active-controlled (except one) Phase III and IV studies were analyzed using descriptive statistics.
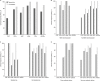
Results: A total of 174 microbiologically evaluable patients with Acinetobacter spp. infections (including MDR infections) were identified, and 95 received tigecycline to treat community-acquired pneumonia (CAP), diabetic foot infections (DFIs), hospital-acquired pneumonia (HAP), complicated intra-abdominal infections (cIAIs), infections with resistant pathogens (RPs), or complicated skin and skin-structure infections. The rate of cure of tigecycline for most indications was 70%-80%, with the highest (88.2%) in cIAIs. The rate of cure of the comparators was generally higher than tigecycline, but within each indication the 95% CIs for clinical cure for each treatment group overlapped. For most Acinetobacter isolates, the minimum inhibitory concentration of tigecycline was 0.12-2 μg/mL, with seven at 4 μg/mL and one at 8 μg/mL. The cure rate by tigecycline was 50% (95% CI 12.5%-87.5% in CAP) to 88.2% (95% CI 66.2%-97.1% in cIAIs) for all Acinetobacter, and 72.7% (95% CI 54.5%-93.2% in HAP) to 100% (95% CI 25%-100.0% in cIAIs) for MDR Acinetobacter. For the comparators, it was 83.8% (95% CI 62.8%-95.9% in HAP) to 100% (95% CI 75%-100% in cIAIs and 25%-100.0% in RPs) and 88% (95% CI 66%-97% in HAP) to 100% (95% CI 25%-100% in cIAIs and 75%-100% in DFIs), respectively.
Conclusion: These findings suggest that with appropriate monitoring, tigecycline may be a useful consideration for Acinetobacter infections alone or in combination with other anti-infective agents when other therapies are not suitable.
Keywords: Acinetobacter; community-acquired pneumonia; complicated intra-abdominal infections; complicated skin and skin-structure infections; tigecycline.
Conflict of interest statement
Disclosure HT, MW, and AQ are employees of Pfizer. AG was an employee of Pfizer at the time the study was conducted. This study used data from clinical trials that have been published previously. Information related to trial registration can be found in the original publications cited as references. The authors report no other conflicts of interest in this work.
Figures
References
-
- World Health Organization Antimicrobial resistance. 2016. [Accessed April 12, 2017]. Available from: http://www.who.int/mediacentre/factsheets/fs194/en.
-
- Centers for Disease Control and Prevention Acinetobacter in healthcare settings. 2010. [Accessed April 12, 2017]. Available from: https://www.cdc.gov/HAI/organisms/acinetobacter.html.
-
- Göttig S, Gruber TM, Higgins PG, Wachsmuth M, Seifert H, Kempf VA. Detection of pan drug-resistant Acinetobacter baumannii in Germany. J Antimicrob Chemother. 2014;69:2578–2579. - PubMed
-
- US Food and Drug Administration Tygacil [prescribing information] 2016. [Accessed April 12, 2017]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021821s039lbl.pdf.
-
- Bouchillon SK, Hoban DJ, Johnson BM, Johnson JL, Hsiung A, Dowzicky MJ. In vitro activity of tigecycline against 3989 Gram-negative and Gram-positive clinical isolates from the United States Tigecycline Evaluation and Surveillance Trial (test program; 2004) Diagn Microbiol Infect Dis. 2005;52:173–179. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

