Vitamin D receptor activation influences the ERK pathway and protects against neurological deficits and neuronal death
- PMID: 29138801
- PMCID: PMC5746295
- DOI: 10.3892/ijmm.2017.3249
Vitamin D receptor activation influences the ERK pathway and protects against neurological deficits and neuronal death
Abstract
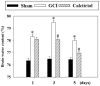
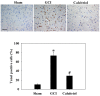
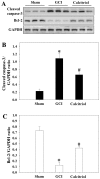
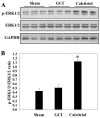
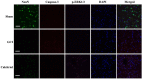
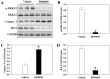
Previous studies have demonstrated that global cerebral ischemia (GCI) causes neurological deficits and neuronal cell apoptosis. Calcitriol, a biologically active metabolite of vitamin D, exerts its endocrinological influence via nuclear vitamin D receptor. It is being assessed as an emerging therapeutic strategy in models of various medical conditions, including acute brain injury. The purpose of the present study was to investigate the neuroprotective effects of calcitriol on GCI and further refine the potential underlying mechanisms. A total of 145 male rats were assigned to 5 groups as follows: Sham group, GCI group, calcitriol treatment group, PD98059 treatment group and vehicle-treated group. Brain water content and neurologic severity score were assessed to evaluate the brain edema and neurological deficits of rats. Histopathological changes and ultrastructures of cells were observed via hematoxylin and eosin stain and transmission electron microscopy, respectively. Immunofluorescent staining and western blot analysis were used to assess the expression of proteins and their co-localization at the molecular level. The results demonstrated that post-GCI administration of calcitriol attenuated brain edema and improved neurological function in rats. Calcitriol also caused marked extracellular signal-regulated kinase 1/2 pathway activation, and thereby attenuated neuronal apoptosis. The present study provided novel clues for understanding the mechanisms by which calcitriol exerts its neuroprotective activity in a rat model of GCI.
Figures




References
-
- Hellman P, Liu W, Westin G, Törmä H, Akerström G. Vitamin D and retinoids in parathyroid glands (Review) Int J Mol Med. 1999;3:355–361. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

