In vitro investigation of the mechanism underlying the effect of ginsenoside on the proliferation and differentiation of neural stem cells subjected to oxygen-glucose deprivation/reperfusion
- PMID: 29138802
- PMCID: PMC5746305
- DOI: 10.3892/ijmm.2017.3253
In vitro investigation of the mechanism underlying the effect of ginsenoside on the proliferation and differentiation of neural stem cells subjected to oxygen-glucose deprivation/reperfusion
Abstract
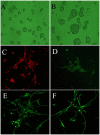
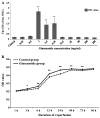
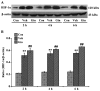
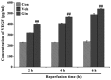
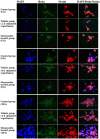
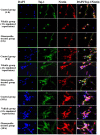
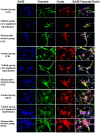
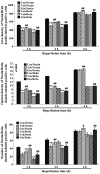
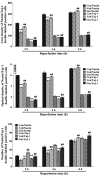
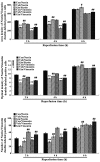
The present study comprised a series of experiments to investigate the mechanism underlying the effect of ginsenoside on the self-renewal, proliferation and differentiation of neural stem cells (NSCs) undergoing oxygen-glucose deprivation/reperfusion (OGD/R) in vitro. The NSCs, which were isolated from the hippocampus of embryonic day 17 embryo rats, were subjected to OGD/R to establish an in vitro model of brain ischemia-reperfusion, following which different doses of ginsenoside were administered to the model. The proliferation of the NSCs was determined using MTT colorimetry and nestin/bromodeoxyuridine (BrdU) immunofluorescent double-labeling. The NSCs were identified by measuring the expression of nestin, and the differentiation of NSCs was assessed through the immunofluorescent double-labeling of nestin/vimentin and nestin/neuron-specific class III β-tubulin (tuj-1). The protein levels of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α) were detected to investigate the function and mechanism of ginsenoside on ischemic stroke using an enzyme-linked immunosorbent assay. Marked increases in the optical density, area density and numbers of nestin/BrdU-, nestin/vimentin- and nestin/tuj-1-positive cells were found in the ginsenoside-treated group. Compared with the control group, enhanced expression levels of BrdU, tuj-1 and vimentin were found in the ginsenoside-treated group, suggesting that ginsenoside may significantly promote the proliferation and differentiation of NSCs. The results of the present study also showed that ginsenoside significantly increased the protein level of HIF-1α (P<0.05) in the NSCs exposed to OGD/R. These results indicated that ginsenoside may maintain NSC replication, promote NSC proliferation and promote NSC differentiation into neurons and astrocytes. Ginsenoside may initiate the expression of downstream VEGF, which is involved in promoting the survival, self-renewal and differentiation of NSCs.
Figures
References
-
- Shi Q, Zhang P, Zhang J, Chen X, Lu H, Tian Y, Parker TL, Liu Y. Adenovirus-mediated brain-derived neurotrophic factor expression regulated by hypoxia response element protects brain from injury of transient middle cerebral artery occlusion in mice. Neurosci Lett. 2009;465:220–225. doi: 10.1016/j.neulet.2009.08.049. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

