SUMO1/UBC9‑decreased Nox1 activity inhibits reactive oxygen species generation and apoptosis in diabetic retinopathy
- PMID: 29138839
- PMCID: PMC5780112
- DOI: 10.3892/mmr.2017.8037
SUMO1/UBC9‑decreased Nox1 activity inhibits reactive oxygen species generation and apoptosis in diabetic retinopathy
Abstract
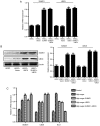
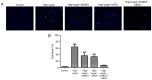
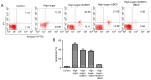
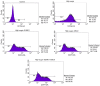
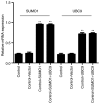
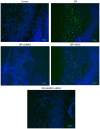
Diabetic retinopathy (DR) is an increasing global health concern that causes vision loss and blindness. Reactive oxygen species (ROS) are considered to be a principal cause of DR. An important source of ROS is the oxidization of NADPH. In the present study, NADPH oxidase 1 (Nox1)‑expressing human retinal epithelial cell (HREC) lines were generated and infected with small ubiquitin‑like modifier 1 (SUMO1) and/or ubiquitin conjugating enzyme E2 I (UBC9) lentiviral pGMLV constructs. The viabilities, apoptotic capacities and ROS production levels of the HREC lines were quantified using Hoechst 33258, annexin V/propidium iodide and dichlorodihydrofluorescein diacetate assays, respectively. Additionally, rat DR models were established. From these models, the apoptotic capacities of retinal tissues were visualized using terminal deoxynucleotidyl transferase dUTP nick end labeling assays, and the pathologies were evaluated. The mRNA and protein expression levels of SUMO1, UBC9 and Nox1 were analyzed using reverse transcription‑quantitative polymerase chain reaction and western blot analyses, respectively. Compared with controls, the relative mRNA levels of SUMO1 and UBC9 were significantly upregulated, and the Nox1 levels significantly downregulated, in cells infected with SUMO1 or UBC9 alone or in combination. The ROS production and apoptosis rates of cells and retinal tissues were decreased. In addition, pathological symptoms in DR tissues improved when they were simultaneously transfected with SUMO1 and UBC9 via intraocular injection. In conclusion, the SUMO1/UBC9 axis may regulate Nox1‑mediated DR by inhibiting ROS generation and apoptosis in rat and cellular model systems.
Figures

Similar articles
-
SUMO1 negatively regulates reactive oxygen species production from NADPH oxidases.Arterioscler Thromb Vasc Biol. 2011 Jul;31(7):1634-42. doi: 10.1161/ATVBAHA.111.226621. Epub 2011 Apr 28. Arterioscler Thromb Vasc Biol. 2011. PMID: 21527745 Free PMC article.
-
Effect of endosulfan and bisphenol A on the expression of SUMO and UBC9.Drug Chem Toxicol. 2020 Nov;43(6):637-644. doi: 10.1080/01480545.2018.1526179. Epub 2018 Nov 14. Drug Chem Toxicol. 2020. PMID: 30426790
-
Interaction between Brucella melitensis 16M and small ubiquitin-related modifier 1 and E2 conjugating enzyme 9 in mouse RAW264.7 macrophages.J Vet Sci. 2019 Sep;20(5):e54. doi: 10.4142/jvs.2019.20.e54. J Vet Sci. 2019. PMID: 31565897 Free PMC article.
-
Mutual enhancement between high-mobility group box-1 and NADPH oxidase-derived reactive oxygen species mediates diabetes-induced upregulation of retinal apoptotic markers.J Physiol Biochem. 2015 Sep;71(3):359-72. doi: 10.1007/s13105-015-0416-x. Epub 2015 Jun 4. J Physiol Biochem. 2015. PMID: 26040511
-
Targeting human 8-oxoguanine DNA glycosylase to mitochondria protects cells from high glucose-induced apoptosis.Endocrine. 2018 Jun;60(3):445-457. doi: 10.1007/s12020-018-1575-7. Epub 2018 Mar 21. Endocrine. 2018. PMID: 29564753
Cited by
-
Schizandrin A Protects Human Retinal Pigment Epithelial Cell Line ARPE-19 against HG-Induced Cell Injury by Regulation of miR-145.Mol Ther Nucleic Acids. 2020 Mar 6;19:42-49. doi: 10.1016/j.omtn.2019.10.026. Epub 2019 Oct 31. Mol Ther Nucleic Acids. 2020. Retraction in: Mol Ther Nucleic Acids. 2022 May 13;28:639. doi: 10.1016/j.omtn.2022.05.015. PMID: 31794890 Free PMC article. Retracted.
-
Diabetic Complications and Oxidative Stress: A 20-Year Voyage Back in Time and Back to the Future.Antioxidants (Basel). 2021 May 5;10(5):727. doi: 10.3390/antiox10050727. Antioxidants (Basel). 2021. PMID: 34063078 Free PMC article. Review.
-
Nitro aspirin (NCX4040) induces apoptosis in PC3 metastatic prostate cancer cells via hydrogen peroxide (H2O2)-mediated oxidative stress.Free Radic Biol Med. 2019 Nov 1;143:494-509. doi: 10.1016/j.freeradbiomed.2019.08.025. Epub 2019 Aug 22. Free Radic Biol Med. 2019. PMID: 31446057 Free PMC article.
-
SUMOylation of Vps34 by SUMO1 promotes phenotypic switching of vascular smooth muscle cells by activating autophagy in pulmonary arterial hypertension.Pulm Pharmacol Ther. 2019 Apr;55:38-49. doi: 10.1016/j.pupt.2019.01.007. Epub 2019 Jan 28. Pulm Pharmacol Ther. 2019. PMID: 30703554 Free PMC article.
-
NADPH oxidases: redox regulation of cell homeostasis and disease.Physiol Rev. 2025 Jul 1;105(3):1291-1428. doi: 10.1152/physrev.00034.2023. Epub 2025 Jan 15. Physiol Rev. 2025. PMID: 39814410 Free PMC article. Review.
References
-
- Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL. The wisconsin epidemiologic study of diabetic retinopathy: II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. JAMA Ophthalmol. 1984;102:520–526. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

