Categorical improvement in functional impairment in depressed patients treated with desvenlafaxine
- PMID: 29140227
- PMCID: PMC6676643
- DOI: 10.1017/S1092852917000633
Categorical improvement in functional impairment in depressed patients treated with desvenlafaxine
Abstract
Objective: This post-hoc pooled analysis evaluated categorical change in functional impairment in patients with major depressive disorder (MDD) treated with desvenlafaxine versus placebo and examined whether early improvement in functioning predicted functional outcomes at study endpoint.
Methods: Data were pooled from eight randomized, double-blind, placebo-controlled studies of desvenlafaxine for the treatment of MDD, including adults who were randomly assigned to receive desvenlafaxine 50 or 100 mg/d or placebo (N=3,384). Shift tables were generated for categorical changes in functional impairment from baseline based on Sheehan Disability Scale (SDS) subscale scores. The categories were none/mild (0-3), moderate (4-6), and marked/extreme (7-10). Treatment comparisons for prespecified shifts of interest and predictive value of week 2 or 4 improvement in SDS subscale scores for functional outcome at week 8 were assessed using logistic regression.
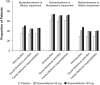
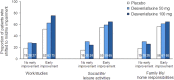
Results: Greater proportions of patients receiving desvenlafaxine 50 and 100 mg achieved improvement from baseline to week 8 for each prespecified shift endpoint versus placebo (all p ≤ 0.02). Early improvement in SDS subscale scores was a statistically significant predictor of functional outcome at week 8, both overall and for each treatment group (all p<0.0001).
Conclusions: Treatment with desvenlafaxine 50 or 100 mg/d led to significantly greater categorical improvement in functional impairment versus placebo, and improvement in SDS subscale scores significantly predicted functional outcome. Monitoring patient progress early in the course of antidepressant treatment using a functional assessment such as the SDS may help clinicians determine whether or not treatment adjustments are needed.
Trial registration: ClinicalTrials.gov NCT00072774 NCT00277823 NCT00300378 NCT00384033 NCT00798707 NCT00863798 NCT01121484 NCT00824291.
Keywords: Antidepressants; Sheehan Disability Scale; functional outcomes; major depressive disorder; treatment outcome.
Figures


References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013: 160–168.
-
- Jain G, Roy A, Harikrishnan V, Yu S, Dabbous O, Lawrence C. Patient-reported depression severity measured by the PHQ–9 and impact on work productivity: results from a survey of full-time employees in the United States. J Occup Environ Med. 2013; 55(3): 252–258. - PubMed
-
- Asami Y, Goren A, Okumura Y. Work productivity loss with depression, diagnosed and undiagnosed, among workers in an internet-based survey conducted in Japan. J Occup Environ Med. 2015; 57(1): 105–110. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

