Safety and Immunogenicity of Different Formulations of Norovirus Vaccine Candidate in Healthy Adults: A Randomized, Controlled, Double-Blind Clinical Trial
- PMID: 29140444
- PMCID: PMC5853300
- DOI: 10.1093/infdis/jix572
Safety and Immunogenicity of Different Formulations of Norovirus Vaccine Candidate in Healthy Adults: A Randomized, Controlled, Double-Blind Clinical Trial
Abstract
Background: We investigated safety and immunogenicity of 1-2 doses of different bivalent virus-like particle (VLP) norovirus vaccine candidate (NoV) formulations in healthy 18- to 64-year-olds.
Methods: On days 1 and 28, participants (n = 420) randomized to 14 equal groups received intramuscular control vaccine (hepatitis A) or 1 of 11 NoV formulations containing varying dosages of GI.1 and GII.4c genotype VLP antigens with aluminum hydroxide [Al(OH)3], and 0 μg, 15 μg, or 50 μg monophosphoryl lipid A (MPL). Immunogenicity was assessed on days 1, 28, 56, 208 and 393. Solicited local and systemic reactions were recorded for 7 days, unsolicited adverse events (AEs) until day 56, and serious AEs throughout the trial.
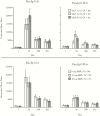
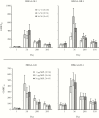
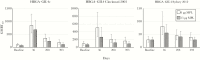
Results: All NoV formulations induced similar increases in pan-immunoglobulin, immunoglobulin A, and histo-blood group binding antigen-blocking antibodies by day 56, mostly after 1 dose, that persisted above baseline to day 393. Higher GI.1 content interfered with GII.4c responses, and responses did not benefit from MPL. Overall reactogenicity consisted of mainly mild injection site pain, headache, and fatigue. No vaccine-related serious AEs were reported.
Conclusions: All candidate NoV formulations were well tolerated. Overall, 15 μg GI.1/50 μg GII.4c elicited the best balance of immunogenicity with no clear benefit of MPL, and is the candidate formulation being taken forward in clinical development.
Clinical trials registration: NCT02038907.
Keywords: adults; immunogenicity; norovirus; reactogenicity; vaccine.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- Foodborne Disease Burden Epidemiology Reference Group 2007–2015. WHO estimates of the global burden of foodborne diseases. http://www.who.int/foodsafety/publications/foodborne_disease/fergreport/en/. Accessed 3 December 2016.
-
- Mattner F, Sohr D, Heim A, Gastmeier P, Vennema H, Koopmans M. Risk groups for clinical complications of norovirus infections: an outbreak investigation. Clin Microbiol Infect 2006; 12:69–74. - PubMed
-
- Trivedi TK, Desai R, Hall AJ, Patel M, Parashar UD, Lopman BA. Clinical characteristics of norovirus-associated deaths: a systematic literature review. Am J Infect Control 2013; 41:654–7. - PubMed
-
- Adler JL, Zickl R. Winter vomiting disease. J Infect Dis 1969; 119:668–73. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

