Selecting an HIV Test: A Narrative Review for Clinicians and Researchers
- PMID: 29140890
- PMCID: PMC5718364
- DOI: 10.1097/OLQ.0000000000000719
Selecting an HIV Test: A Narrative Review for Clinicians and Researchers
Abstract
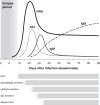
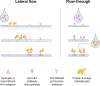
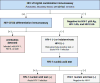
Given the many options available, selecting an HIV test for a particular clinical or research setting can be daunting. Making an informed decision requires an assessment of the likelihood of acute infection in the test population and an understanding of key aspects of the tests themselves. The ability of individual tests to reliably detect HIV infection depends on the target(s) being detected, when they can be expected to be present after infection, and the concentration of stable target in test specimens, all of which are explained by the virologic and serologic events after infection. The purpose of this article is to review the timeline of HIV infection, nomenclature, and characteristics of different tests; compare point-of-care and laboratory-based tests; discuss the impact of different specimens on test performance; and provide practical advice to help clinicians and researchers new to the field select a test that best suits their needs.
Figures

Comment in
-
The Evolution of HIV Testing Continues.Sex Transm Dis. 2017 Dec;44(12):747-749. doi: 10.1097/OLQ.0000000000000736. Sex Transm Dis. 2017. PMID: 29140891 Free PMC article. No abstract available.
References
-
- Branson BM. HIV testing updates and challenges: when regulatory caution and public health imperatives collide. Current HIV/AIDS reports. 2015;12(1):117–126. - PubMed
-
- Delaney KP, Hanson DL, Masciotra S, Ethridge SF, Wesolowski L, Owen SM. Time until emergence of HIV test reactivity following infection with HIV-1: implications for interpreting test results and retesting after exposure. Clin Infect Dis. 2017;64(1):53–59. - PubMed
-
- Busch MP, Satten GA. Time course of viremia and antibody seroconversion following human immunodeficiency virus exposure. Am J Med. 1997;102(5B):117–124. discussion 125–116. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

