Fingolimod reduces neuropathic pain behaviors in a mouse model of multiple sclerosis by a sphingosine-1 phosphate receptor 1-dependent inhibition of central sensitization in the dorsal horn
- PMID: 29140922
- PMCID: PMC6287931
- DOI: 10.1097/j.pain.0000000000001106
Fingolimod reduces neuropathic pain behaviors in a mouse model of multiple sclerosis by a sphingosine-1 phosphate receptor 1-dependent inhibition of central sensitization in the dorsal horn
Abstract
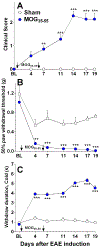
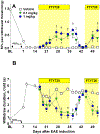
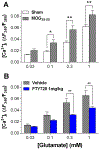
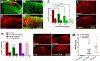
Multiple sclerosis (MS) is an autoimmune-inflammatory neurodegenerative disease that is often accompanied by a debilitating neuropathic pain. Disease-modifying agents slow down the progression of multiple sclerosis and prevent relapses, yet it remains unclear if they yield analgesia. We explored the analgesic potential of fingolimod (FTY720), an agonist and/or functional antagonist at the sphingosine-1-phosphate receptor 1 (S1PR1), because it reduces hyperalgesia in models of peripheral inflammatory and neuropathic pain. We used a myelin oligodendrocyte glycoprotein 35 to 55 (MOG35-55) mouse model of experimental autoimmune encephalomyelitis, modified to avoid frank paralysis, and thus, allow for assessment of withdrawal behaviors to somatosensory stimuli. Daily intraperitoneal fingolimod reduced behavioral signs of central neuropathic pain (mechanical and cold hypersensitivity) in a dose-dependent and reversible manner. Both autoimmune encephalomyelitis and fingolimod changed hyperalgesia before modifying motor function, suggesting that pain-related effects and clinical neurological deficits were modulated independently. Fingolimod also reduced cellular markers of central sensitization of neurons in the dorsal horn of the spinal cord: glutamate-evoked Ca signaling and stimulus-evoked phospho-extracellular signal-related kinase ERK (pERK) expression, as well as upregulation of astrocytes (GFAP) and macrophage/microglia (Iba1) immunoreactivity. The antihyperalgesic effects of fingolimod were prevented or reversed by the S1PR1 antagonist W146 (1 mg/kg daily, i.p.) and could be mimicked by either repeated or single injection of the S1PR1-selective agonist SEW2871. Fingolimod did not change spinal membrane S1PR1 content, arguing against a functional antagonist mechanism. We conclude that fingolimod behaves as an S1PR1 agonist to reduce pain in multiple sclerosis by reversing central sensitization of spinal nociceptive neurons.
Figures




Similar articles
-
Dissociation between the anti-allodynic effects of fingolimod (FTY720) and desensitization of S1P1 receptor-mediated G-protein activation in a mouse model of sciatic nerve injury.Neuropharmacology. 2024 Dec 15;261:110165. doi: 10.1016/j.neuropharm.2024.110165. Epub 2024 Sep 18. Neuropharmacology. 2024. PMID: 39303855
-
The development and maintenance of paclitaxel-induced neuropathic pain require activation of the sphingosine 1-phosphate receptor subtype 1.J Biol Chem. 2014 Jul 25;289(30):21082-97. doi: 10.1074/jbc.M114.569574. J Biol Chem. 2014. PMID: 24876379 Free PMC article.
-
Differential Tolerance to FTY720-Induced Antinociception in Acute Thermal and Nerve Injury Mouse Pain Models: Role of Sphingosine-1-Phosphate Receptor Adaptation.J Pharmacol Exp Ther. 2018 Sep;366(3):509-518. doi: 10.1124/jpet.118.248260. Epub 2018 Jun 26. J Pharmacol Exp Ther. 2018. PMID: 29945931 Free PMC article.
-
Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy.J Neurol Sci. 2013 May 15;328(1-2):9-18. doi: 10.1016/j.jns.2013.02.011. Epub 2013 Mar 19. J Neurol Sci. 2013. PMID: 23518370 Free PMC article. Review.
-
Mechanisms of fingolimod's efficacy and adverse effects in multiple sclerosis.Ann Neurol. 2011 May;69(5):759-77. doi: 10.1002/ana.22426. Ann Neurol. 2011. PMID: 21520239 Review.
Cited by
-
Targeting the Sphingosine-1-Phosphate Axis for Developing Non-narcotic Pain Therapeutics.Trends Pharmacol Sci. 2020 Nov;41(11):851-867. doi: 10.1016/j.tips.2020.09.006. Epub 2020 Oct 1. Trends Pharmacol Sci. 2020. PMID: 33010954 Free PMC article. Review.
-
Laparoscopic adrenalectomy: lateral transperitoneal versus posterior retroperitoneal approach - prospective randomized trial.Wideochir Inne Tech Maloinwazyjne. 2019 Apr;14(2):160-169. doi: 10.5114/wiitm.2019.84694. Epub 2019 May 5. Wideochir Inne Tech Maloinwazyjne. 2019. PMID: 31118978 Free PMC article.
-
Preparation and In vitro/In vivo Evaluation of Fingolimod hydrochloride Loaded Polymeric Mixed Nano-Micelles for Treatment of Multiple Sclerosis.J Neuroimmune Pharmacol. 2025 Apr 16;20(1):41. doi: 10.1007/s11481-025-10203-8. J Neuroimmune Pharmacol. 2025. PMID: 40237870
-
Neuropathic-like Nociception and Spinal Cord Neuroinflammation Are Dependent on the TRPA1 Channel in Multiple Sclerosis Models in Mice.Cells. 2023 May 30;12(11):1511. doi: 10.3390/cells12111511. Cells. 2023. PMID: 37296632 Free PMC article.
-
Dissociation between the anti-allodynic effects of fingolimod (FTY720) and desensitization of S1P1 receptor-mediated G-protein activation in a mouse model of sciatic nerve injury.Neuropharmacology. 2024 Dec 15;261:110165. doi: 10.1016/j.neuropharm.2024.110165. Epub 2024 Sep 18. Neuropharmacology. 2024. PMID: 39303855
References
-
- Aicher SA, Silverman MB, Winkler CW, Bebo BF Jr. Hyperalgesia in an animal model of multiple sclerosis. Pain 2004;110(3):560–570. - PubMed
-
- Akiyama T, Sadahira Y, Matsubara K, Mori M, Igarashi Y. Immunohistochemical detection of sphingosine-1-phosphate receptor 1 in vascular and lymphatic endothelial cells. J Mol Histol 2008;39(5):527–533. - PubMed
-
- Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nature reviews Immunology 2007;7(11):904–912. - PubMed
-
- Begum F, Zhu W, Cortes C, MacNeil B, Namaka M. Elevation of tumor necrosis factor alpha in dorsal root ganglia and spinal cord is associated with neuroimmune modulation of pain in an animal model of multiple sclerosis. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 2013;8(3):677–690. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

