T-SPOT.TB Performance in Routine Pediatric Practice in a Low TB Burden Setting
- PMID: 29140933
- PMCID: PMC12190735
- DOI: 10.1097/INF.0000000000001792
T-SPOT.TB Performance in Routine Pediatric Practice in a Low TB Burden Setting
Abstract
Background: The T-SPOT.TB, an interferon-gamma release assay, is an indirect test of Mycobacterium tuberculosis infection. Due to sparse and conflicting evidence, the use of interferon-gamma release assay is limited in young and HIV-infected children. We determined the prevalence of invalid, borderline, positive and negative results and associations with key demographic variables during routine pediatric use in a low tuberculosis burden setting.
Methods: For pediatric samples received at Oxford Diagnostic Laboratories between 2010 and 2015, the associations between initial test outcome and demographics were estimated by bivariate analysis and logistic regression.
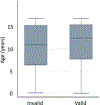
Results: A total of 44,289 samples (median age 12.5 years; interquartile range 7.7-15.5), including 5057 samples (11.6%) from children under 5 years old, were received from 46 U.S. states, Washington, DC and Puerto Rico. A total of 592 samples (1.3%) could not be tested. T-SPOT.TB positivity was strongly correlated (r = 0.60; P < 0.0001) with state TB incidence. Compared with negative results, positive results were more likely in samples from older children (P < 0.0001), public health clinics (P < 0.0001) and rural locations (P = 0.005). Although infrequent (0.6%), invalid results were more common in samples collected at HIV clinics (odds ratio = 2.5, 95% confidence interval: 1.3-4.9) and from younger children (P = 0.03). These invalid results were more likely due to a robust nil (negative) control response rather than a weak mitogen (positive) control response.
Conclusions: The T-SPOT.TB test correlated strongly with well-recognized risk factors for tuberculosis infection and provided evaluable results in 98% of children. To optimize the impact of testing on clinical decision making and patient outcomes, local epidemiology and individual patient risk should be considered when incorporating IGRAs into pediatric guidelines.
Conflict of interest statement
The other authors have no conflicts of interest to disclose.
Figures

References
-
- WHO. Global Tuberculosis Report, 2016. Geneva, Switzerland: WHO; 2016. Contract No.: WHO/HTM/TB/2016.13.
-
- Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis. 2016;16:1193–1201. - PubMed
-
- Uplekar M, Weil D, Lonnroth K, et al. ; for WHO’s Global TB Programme. WHO’s new end TB strategy. Lancet. 2015;385:1799–1801. - PubMed
-
- Mack U, Migliori GB, Sester M, et al. ; C. Lange; TBNET. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J. 2009;33:956–973. - PubMed
-
- Andersen P, Munk ME, Pollock JM, et al. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099–1104. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

