A coupled-oscillator model of olfactory bulb gamma oscillations
- PMID: 29140973
- PMCID: PMC5706731
- DOI: 10.1371/journal.pcbi.1005760
A coupled-oscillator model of olfactory bulb gamma oscillations
Abstract
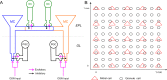
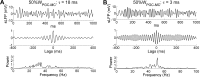
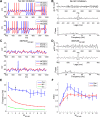
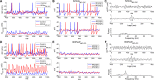
The olfactory bulb transforms not only the information content of the primary sensory representation, but also its underlying coding metric. High-variance, slow-timescale primary odor representations are transformed by bulbar circuitry into secondary representations based on principal neuron spike patterns that are tightly regulated in time. This emergent fast timescale for signaling is reflected in gamma-band local field potentials, presumably serving to efficiently integrate olfactory sensory information into the temporally regulated information networks of the central nervous system. To understand this transformation and its integration with interareal coordination mechanisms requires that we understand its fundamental dynamical principles. Using a biophysically explicit, multiscale model of olfactory bulb circuitry, we here demonstrate that an inhibition-coupled intrinsic oscillator framework, pyramidal resonance interneuron network gamma (PRING), best captures the diversity of physiological properties exhibited by the olfactory bulb. Most importantly, these properties include global zero-phase synchronization in the gamma band, the phase-restriction of informative spikes in principal neurons with respect to this common clock, and the robustness of this synchronous oscillatory regime to multiple challenging conditions observed in the biological system. These conditions include substantial heterogeneities in afferent activation levels and excitatory synaptic weights, high levels of uncorrelated background activity among principal neurons, and spike frequencies in both principal neurons and interneurons that are irregular in time and much lower than the gamma frequency. This coupled cellular oscillator architecture permits stable and replicable ensemble responses to diverse sensory stimuli under various external conditions as well as to changes in network parameters arising from learning-dependent synaptic plasticity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures











References
-
- Shepherd GM, Chen WR, Greer CA. Olfactory bulb In: The Synaptic Organization of the Brain (5th ed.), edited by Shepherd GM. Oxford, UK: Oxford Univ. Press; 2004. pp. 165–216.
-
- Cleland TA. Construction of odor representations by olfactory bulb microcircuits. Prog Brain Res. 2014; 208:177–203. doi: 10.1016/B978-0-444-63350-7.00007-3 - DOI - PubMed
-
- Kay LM, Lazzara P. How global are olfactory bulb oscillations? J Neurophysiol. 2010; 104(3):1768–1773. doi: 10.1152/jn.00478.2010 - DOI - PMC - PubMed
-
- Freeman WJ, Viana Di Prisco G. Relation of olfactory EEG to behavior: time series analysis. Behav Neurosci. 1986; 100(5):753–763. - PubMed
-
- Freeman WJ, Baird B. Relation of olfactory EEG to behavior: spatial analysis. Behav Neurosci. 1987; 101(3):393–408. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

