Suppression of inflammatory and infection responses in lung macrophages by eucalyptus oil and its constituent 1,8-cineole: Role of pattern recognition receptors TREM-1 and NLRP3, the MAP kinase regulator MKP-1, and NFκB
- PMID: 29141025
- PMCID: PMC5687727
- DOI: 10.1371/journal.pone.0188232
Suppression of inflammatory and infection responses in lung macrophages by eucalyptus oil and its constituent 1,8-cineole: Role of pattern recognition receptors TREM-1 and NLRP3, the MAP kinase regulator MKP-1, and NFκB
Abstract
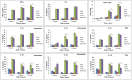
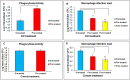
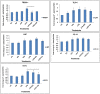
Eucalyptus oil (EO) used in traditional medicine continues to prove useful for aroma therapy in respiratory ailments; however, there is a paucity of information on its mechanism of action and active components. In this direction, we investigated EO and its dominant constituent 1,8-cineole (eucalyptol) using the murine lung alveolar macrophage (AM) cell line MH-S. In an LPS-induced AM inflammation model, pre-treatment with EO significantly reduced (P ≤0.01or 0.05) the pro-inflammatory mediators TNF-α, IL-1 (α and β), and NO, albeit at a variable rate and extent; 1,8-cineole diminished IL-1 and IL-6. In a mycobacterial-infection AM model, EO pre-treatment or post-treatment significantly enhanced (P ≤0.01) the phagocytic activity and pathogen clearance. 1,8-cineole also significantly enhanced the pathogen clearance though the phagocytic activity was not significantly altered. EO or 1,8-cineole pre-treatment attenuated LPS-induced inflammatory signaling pathways at various levels accompanied by diminished inflammatory response. Among the pattern recognition receptors (PRRs) involved in LPS signaling, the TREM pathway surface receptor (TREM-1) was significantly downregulated. Importantly, the pre-treatments significantly downregulated (P ≤0.01) the intracellular PRR receptor NLRP3 of the inflammasome, which is consistent with the decrease in IL-1β secretion. Of the shared downstream signaling cascade for these PRR pathways, there was significant attenuation of phosphorylation of the transcription factor NF-κB and p38 (but increased phosphorylation of the other two MAP kinases, ERK1/2 and JNK1/2). 1,8-cineole showed a similar general trend except for an opposite effect on NF-κB and JNK1/2. In this context, either pre-treatment caused a significant downregulation of MKP-1 phosphatase, a negative regulator of MAPKs. Collectively, our results demonstrate that the anti-inflammatory activity of EO and 1,8-cineole is modulated via selective downregulation of the PRR pathways, including PRR receptors (TREM-1 and NLRP3) and common downstream signaling cascade partners (NF-κB, MAPKs, MKP-1). To our knowledge, this is the first report on the modulatory role of TREM-1 and NLRP3 inflammasome pathways and the MAPK negative regulator MKP-1 in context of the anti-inflammatory potential of EO and its constituent 1,8-cineole.
Conflict of interest statement
Figures





References
-
- Sarkar S, Mazumder S, Saha SJ, Bandyopadhyay U. Management of inflammation by natural polyphenols: A comprehensive mechanistic update. Curr Med Chem. 2016; 23(16): 1657–1695. - PubMed
-
- Tabutia JRS, Kukundab CB, Waako PJ. Medicinal plants used by traditional medicine practitioners in the treatment of tuberculosis and related ailments in Uganda. J Ethnopharmacol. 2010; 127: 130–136. doi: 10.1016/j.jep.2009.09.035 - DOI - PubMed
-
- Tsai ML, Lin CC, Lin WC, Yang CH. Antimicrobial, antioxidant, and anti-inflammatory activities of essential oils from five selected herbs. Biosci Biotechnol Biochem. 2011; 75(10): 1977–1983. - PubMed
-
- Laird K, Phillips C. Vapor phase: a potential future use for essential oils as antimicrobials. Letters in Appl Microbiol. 2012; 54(3): 169–174. - PubMed
-
- Vuong QV, Chalmers AC, Jyoti Bhuyan D, Bowyer MC, Scarlett CJ. Botanical, phytochemical, and anticancer properties of the Eucalyptus species. Chem Biodivers. 2015; 12(6): 907–924 doi: 10.1002/cbdv.201400327 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

