Lateral Preoptic Control of the Lateral Habenula through Convergent Glutamate and GABA Transmission
- PMID: 29141211
- PMCID: PMC5699228
- DOI: 10.1016/j.celrep.2017.10.066
Lateral Preoptic Control of the Lateral Habenula through Convergent Glutamate and GABA Transmission
Abstract
The lateral habenula (LHb) is a brain structure that participates in cognitive and emotional processing and has been implicated in several mental disorders. Although one of the largest inputs to the LHb originates in the lateral preoptic area (LPO), little is known about how the LPO participates in the regulation of LHb function. Here, we provide evidence that the LPO exerts bivalent control over the LHb through the convergent transmission of LPO glutamate and γ-aminobutyric acid (GABA) onto single LHb neurons. In vivo, both LPO-glutamatergic and LPO-GABAergic inputs to the LHb are activated by aversive stimuli, and their predictive cues yet produce opposing behaviors when stimulated independently. These results support a model wherein the balanced response of converging LPO-glutamate and LPO-GABA are necessary for a normal response to noxious stimuli, and an imbalance in LPO→LHb glutamate or GABA results in the type of aberrant processing that may underlie mental disorders.
Keywords: GABA; aversion; calcium imaging; electron microscopy; glutamate; habenula; optogenetics; preoptic; reward; stress; synapse.
Published by Elsevier Inc.
Conflict of interest statement
Figures


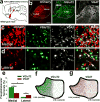

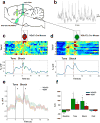
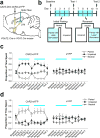
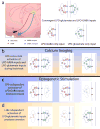
References
-
- Araki M, McGeer P, McGeer E. Retrograde HRP tracing combined with a pharmacohistochemical method for GABA transaminase for the identification of presumptive GABAergic projections to the habenula. Brain Res. 1984;304:271–277. - PubMed
-
- Borgius L, Restrepo CE, Leao RN, Saleh N, Kiehn O. A transgenic mouse line for molecular genetic analysis of excitatory glutamatergic neurons. Mol Cell Neurosci. 2010;45:245–257. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

