Widespread Translational Remodeling during Human Neuronal Differentiation
- PMID: 29141229
- PMCID: PMC5759054
- DOI: 10.1016/j.celrep.2017.10.095
Widespread Translational Remodeling during Human Neuronal Differentiation
Abstract
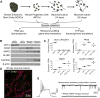
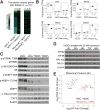
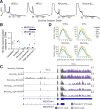
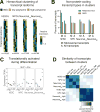
Faithful cellular differentiation requires temporally precise activation of gene expression programs, which are coordinated at the transcriptional and translational levels. Neurons express the most complex set of mRNAs of any human tissue, but translational changes during neuronal differentiation remain incompletely understood. Here, we induced forebrain neuronal differentiation of human embryonic stem cells (hESCs) and measured genome-wide RNA and translation levels with transcript-isoform resolution. We found that thousands of genes change translation status during differentiation without a corresponding change in RNA level. Specifically, we identified mTOR signaling as a key driver for elevated translation of translation-related genes in hESCs. In contrast, translational repression in active neurons is mediated by regulatory sequences in 3' UTRs. Together, our findings identify extensive translational control changes during human neuronal differentiation and a crucial role of 3' UTRs in driving cell-type-specific translation.
Keywords: RNA; TrIP-seq; cellular differentiation; human stem cell; neural progenitor cell; neurogenesis; neuron; polysome profiling; ribosome profiling; translational control.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B, Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR, et al. Role of Sox2 in the development of the mouse neocortex. Dev Biol. 2006;295:52–66. - PubMed
-
- Bhat M, Robichaud N, Hulea L, Sonenberg N, Pelletier J, Topisirovic I. Targeting the translation machinery in cancer. Nat Rev Drug Discov. 2015;14:261–278. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

