Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way
- PMID: 29141446
- PMCID: PMC5955787
- DOI: 10.1080/21691401.2017.1388249
Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way
Abstract
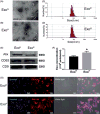
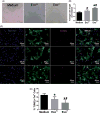
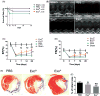
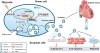
Hypoxia treatment enhances paracrine effect of mesenchymal stem cells (MSCs). The aim of this study was to investigate whether exosomes from hypoxia-treated MSCs (ExoH) are superior to those from normoxia-treated MSCs (ExoN) for myocardial repair. Mouse bone marrow-derived MSCs were cultured under hypoxia or normoxia for 24 h, and exosomes from conditioned media were intramyocardially injected into infarcted heart of C57BL/6 mouse. ExoH resulted in significantly higher survival, smaller scar size and better cardiac functions recovery. ExoH conferred increased vascular density, lower cardiomyocytes (CMs) apoptosis, reduced fibrosis and increased recruitment of cardiac progenitor cells in the infarcted heart relative to ExoN. MicroRNA analysis revealed significantly higher levels of microRNA-210 (miR-210) in ExoH compared with ExoN. Transfection of a miR-210 mimic into endothelial cells (ECs) and CMs conferred similar biological effects as ExoH. Hypoxia treatment of MSCs increased the expression of neutral sphingomyelinase 2 (nSMase2) which is crucial for exosome secretion. Blocking the activity of nSMase2 resulted in reduced miR-210 secretion and abrogated the beneficial effects of ExoH. In conclusion, hypoxic culture augments miR-210 and nSMase2 activities in MSCs and their secreted exosomes, and this is responsible at least in part for the enhanced cardioprotective actions of exosomes derived from hypoxia-treated cells.
Keywords: Exosomes; MSCs; hypoxia; microRNA210; myocardial infarction; nSMase2.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures



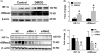
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
