The dose of behavioral interventions to prevent and treat childhood obesity: a systematic review and meta-regression
- PMID: 29141651
- PMCID: PMC5688650
- DOI: 10.1186/s12966-017-0615-7
The dose of behavioral interventions to prevent and treat childhood obesity: a systematic review and meta-regression
Abstract
Background: A better understanding of the optimal "dose" of behavioral interventions to affect change in weight-related outcomes is a critical topic for childhood obesity intervention research. The objective of this review was to quantify the relationship between dose and outcome in behavioral trials targeting childhood obesity to guide future intervention development.
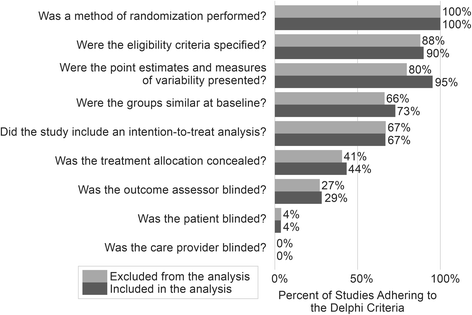
Methods: A systematic review and meta-regression included randomized controlled trials published between 1990 and June 2017 that tested a behavioral intervention for obesity among children 2-18 years old. Searches were conducted among PubMed (Web-based), Cumulative Index to Nursing and Allied Health Literature (EBSCO platform), PsycINFO (Ovid platform) and EMBASE (Ovid Platform). Two coders independently reviewed and abstracted each included study. Dose was extracted as intended intervention duration, number of sessions, and length of sessions. Standardized effect sizes were calculated from change in weight-related outcome (e.g., BMI-Z score).
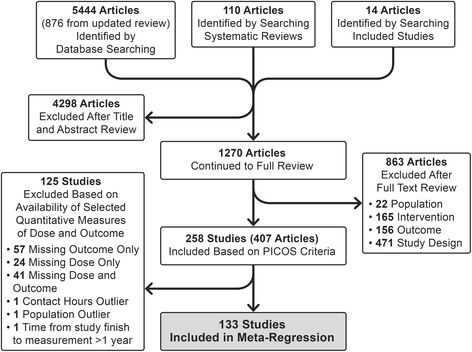
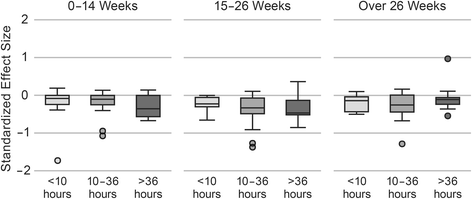
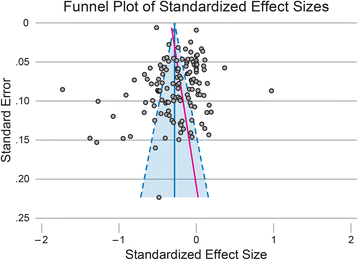
Results: Of the 258 studies identified, 133 had sufficient data to be included in the meta-regression. Average intended total contact (# sessions x length of sessions) was 27.7 (SD 32.2) hours and average duration was 26.0 (SD 23.4) weeks. When controlling for study covariates, a random-effects meta-regression revealed no significant association between contact hours, intended duration or their interaction and effect size.
Conclusions: This systematic review identified wide variation in the dose of behavioral interventions to prevent and treat pediatric obesity, but was unable to detect a clear relationship between dose and weight-related outcomes. There is insufficient evidence to provide quantitative guidance for future intervention development. One limitation of this review was the ability to uniformly quantify dose due to a wide range of reporting strategies. Future trials should report dose intended, delivered, and received to facilitate quantitative evaluation of optimal dose.
Trial registrations: The protocol was registered on PROSPERO (Registration # CRD42016036124 ).
Keywords: Behavioral intervention; Childhood obesity; Dose; Health behavior; Systematic review.
Conflict of interest statement
Ethics approval and consent to participate
This manuscript represents a systematic review of the literature, and was not human subjects research.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Grants and funding
- U01HL103629/HL/NHLBI NIH HHS/United States
- U01 HL103629/HL/NHLBI NIH HHS/United States
- T32 NR015433/NR/NINR NIH HHS/United States
- P30DK092986/DK/NIDDK NIH HHS/United States
- U01 HL103561/HL/NHLBI NIH HHS/United States
- UL1 TR000114/TR/NCATS NIH HHS/United States
- K23 HL127104/HL/NHLBI NIH HHS/United States
- U01HD068890/HL/NHLBI NIH HHS/United States
- U01 HL103620/HL/NHLBI NIH HHS/United States
- U01HL103620/National Heart, Lung, and Blood Institute (US)/International
- P30 DK056350/DK/NIDDK NIH HHS/United States
- K23HL127104/National Heart, Lung, and Blood Institute (US)/International
- U01 HL103622/HL/NHLBI NIH HHS/United States
- UL1TR000114/TR/NCATS NIH HHS/United States
- U01HL103561/HL/NHLBI NIH HHS/United States
- P30 DK092986/DK/NIDDK NIH HHS/United States
- T32 NR015433-01/National Institute of Nursing Research (US)/International
- U01 HD068890/HD/NICHD NIH HHS/United States
- U01HL103622/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

