Local estrogen axis in the human bone microenvironment regulates estrogen receptor-positive breast cancer cells
- PMID: 29141657
- PMCID: PMC5688761
- DOI: 10.1186/s13058-017-0910-x
Local estrogen axis in the human bone microenvironment regulates estrogen receptor-positive breast cancer cells
Abstract
Background: Approximately 70% of all breast cancers express the estrogen receptor, and are regulated by estrogen. While the ovaries are the primary source of estrogen in premenopausal women, most breast cancer is diagnosed following menopause, when systemic levels of this hormone decline. Estrogen production from androgen precursors is catalyzed by the aromatase enzyme. Although aromatase expression and local estrogen production in breast adipose tissue have been implicated in the development of primary breast cancer, the source of estrogen involved in the regulation of estrogen receptor-positive (ER+) metastatic breast cancer progression is less clear.
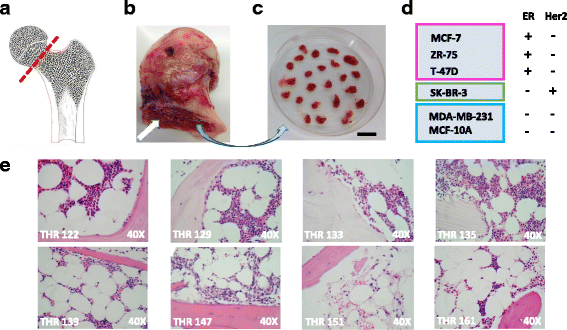
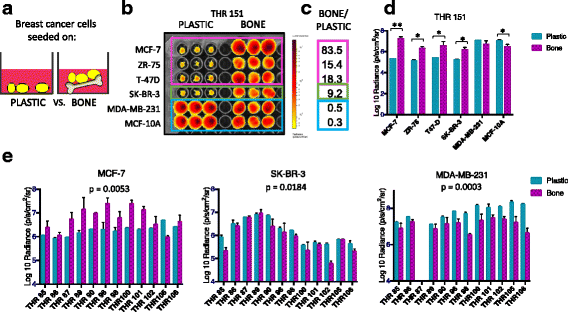
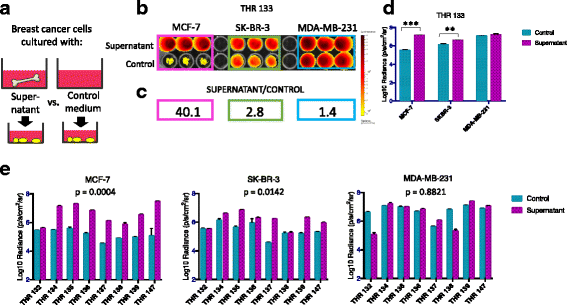
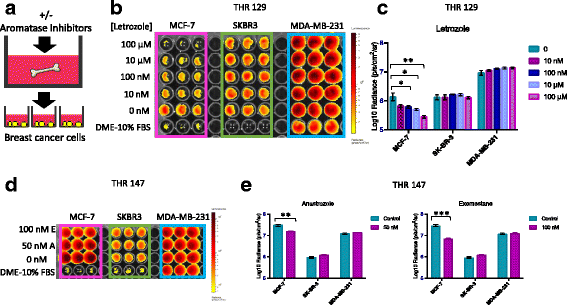
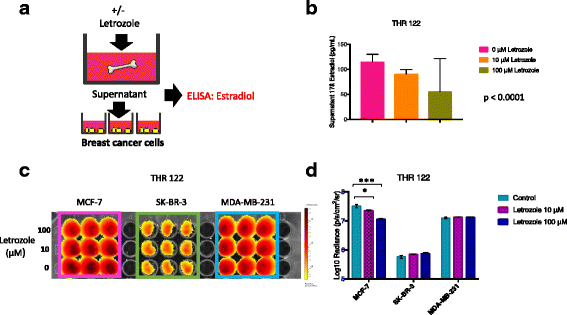
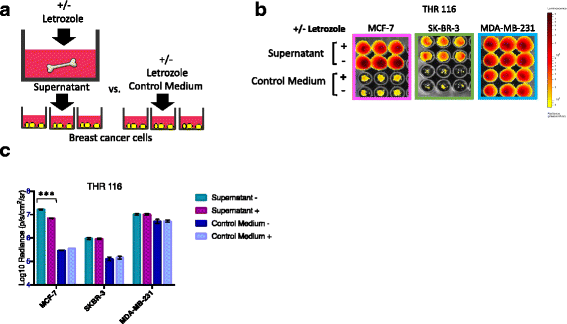
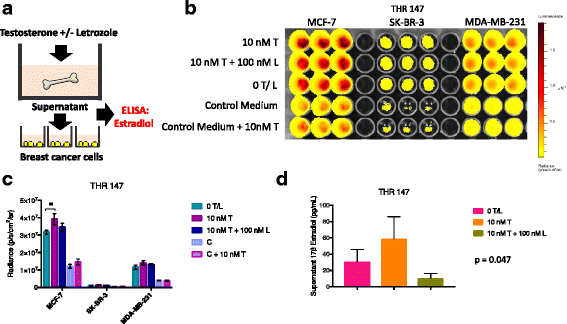
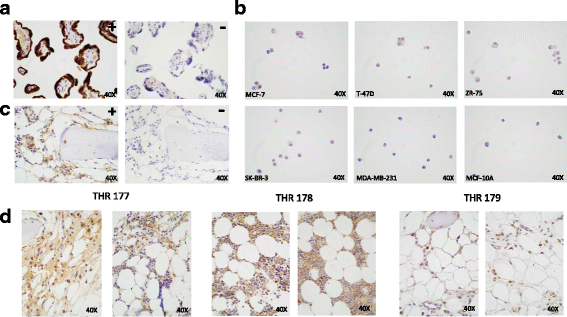
Methods: Bone is the most common distant site of breast cancer metastasis, particularly for ER+ breast cancers. We employed a co-culture model using trabecular bone tissues obtained from total hip replacement (THR) surgery specimens to study ER+ and estrogen receptor-negative (ER-) breast cancer cells within the human bone microenvironment. Luciferase-expressing ER+ (MCF-7, T-47D, ZR-75) and ER- (SK-BR-3, MDA-MB-231, MCF-10A) breast cancer cells were cultured directly on bone tissue fragments or in bone tissue-conditioned media, and monitored over time with bioluminescence imaging (BLI). Bone tissue-conditioned media were generated in the presence vs. absence of aromatase inhibitors, and testosterone. Bone tissue fragments were analyzed for aromatase expression by immunohistochemistry.
Results: ER+ breast cancer cells were preferentially sustained in co-cultures with bone tissues and bone tissue-conditioned media relative to ER- cells. Bone fragments analyzed by immunohistochemistry revealed expression of the aromatase enzyme. Bone tissue-conditioned media generated in the presence of testosterone had increased estrogen levels and heightened capacity to stimulate ER+ breast cancer cell proliferation. Pretreatment of cultured bone tissues with aromatase inhibitors, which inhibited estrogen production, reduced the capacity of conditioned media to stimulate ER+ cell proliferation.
Conclusions: These results suggest that a local estrogen signaling axis regulates ER+ breast cancer cell viability and proliferation within the bone metastatic niche, and that aromatase inhibitors modulate this axis. Although endocrine therapies are highly effective in the treatment of ER+ breast cancer, resistance to these treatments reduces their efficacy. Characterization of estrogen signaling networks within the bone microenvironment will identify new strategies for combating metastatic progression and endocrine resistance.
Keywords: Aromatase; Aromatase inhibitors; Bone tissue culture; Breast cancer metastasis to bone; Estrogen receptor positive breast cancer.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was obtained by the Stanford University Internal Review Board (IRB). All discarded surgical specimens and patient medical record information, including diagnoses and disease history (obtained from the STRIDE database by Research IT, Stanford Medicine), were collected under IRB Protocol # 38625, with a Waiver of Consent, in accordance with regulations of the Stanford University Research Compliance Office.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Tumor-stromal interaction through the estrogen-signaling pathway in human breast cancer.Cancer Res. 2005 Jun 1;65(11):4653-62. doi: 10.1158/0008-5472.CAN-04-3236. Cancer Res. 2005. PMID: 15930283
-
Breast Cancer Cell Colonization of the Human Bone Marrow Adipose Tissue Niche.Neoplasia. 2015 Dec;17(12):849-861. doi: 10.1016/j.neo.2015.11.005. Neoplasia. 2015. PMID: 26696367 Free PMC article.
-
Endogenous aromatization of testosterone results in growth stimulation of the human MCF-7 breast cancer cell line.J Steroid Biochem Mol Biol. 2005 Jan;93(1):25-34. doi: 10.1016/j.jsbmb.2004.11.005. Epub 2005 Jan 27. J Steroid Biochem Mol Biol. 2005. PMID: 15748829
-
Structural and functional characterization of aromatase, estrogen receptor, and their genes in endocrine-responsive and -resistant breast cancer cells.J Steroid Biochem Mol Biol. 2016 Jul;161:73-83. doi: 10.1016/j.jsbmb.2015.07.018. Epub 2015 Aug 13. J Steroid Biochem Mol Biol. 2016. PMID: 26277097 Free PMC article. Review.
-
Estrogen metabolites and breast cancer.Steroids. 2015 Jul;99(Pt A):61-6. doi: 10.1016/j.steroids.2014.08.003. Epub 2014 Aug 26. Steroids. 2015. PMID: 25168343 Review.
Cited by
-
Estrone, the major postmenopausal estrogen, binds ERa to induce SNAI2, epithelial-to-mesenchymal transition, and ER+ breast cancer metastasis.Cell Rep. 2022 Nov 15;41(7):111672. doi: 10.1016/j.celrep.2022.111672. Cell Rep. 2022. PMID: 36384125 Free PMC article.
-
A Comparison of Osteoblast and Osteoclast In Vitro Co-Culture Models and Their Translation for Preclinical Drug Testing Applications.Int J Mol Sci. 2020 Jan 30;21(3):912. doi: 10.3390/ijms21030912. Int J Mol Sci. 2020. PMID: 32019244 Free PMC article. Review.
-
Aromatase Inhibition Eliminates Sexual Receptivity Without Enhancing Weight Gain in Ovariectomized Marmoset Monkeys.J Endocr Soc. 2022 Apr 22;6(6):bvac063. doi: 10.1210/jendso/bvac063. eCollection 2022 Jun 1. J Endocr Soc. 2022. PMID: 35592515 Free PMC article.
-
Molecular insights into the interplay between adiposity, breast cancer and bone metastasis.Clin Exp Metastasis. 2021 Apr;38(2):119-138. doi: 10.1007/s10585-021-10076-0. Epub 2021 Feb 16. Clin Exp Metastasis. 2021. PMID: 33591548 Review.
-
Morphing electronics enable neuromodulation in growing tissue.Nat Biotechnol. 2020 Sep;38(9):1031-1036. doi: 10.1038/s41587-020-0495-2. Epub 2020 Apr 20. Nat Biotechnol. 2020. PMID: 32313193 Free PMC article.
References
-
- Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. 2003;144(10):4562–74. doi: 10.1210/en.2003-0567. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

