Intron retention enhances gene regulatory complexity in vertebrates
- PMID: 29141666
- PMCID: PMC5688624
- DOI: 10.1186/s13059-017-1339-3
Intron retention enhances gene regulatory complexity in vertebrates
Abstract
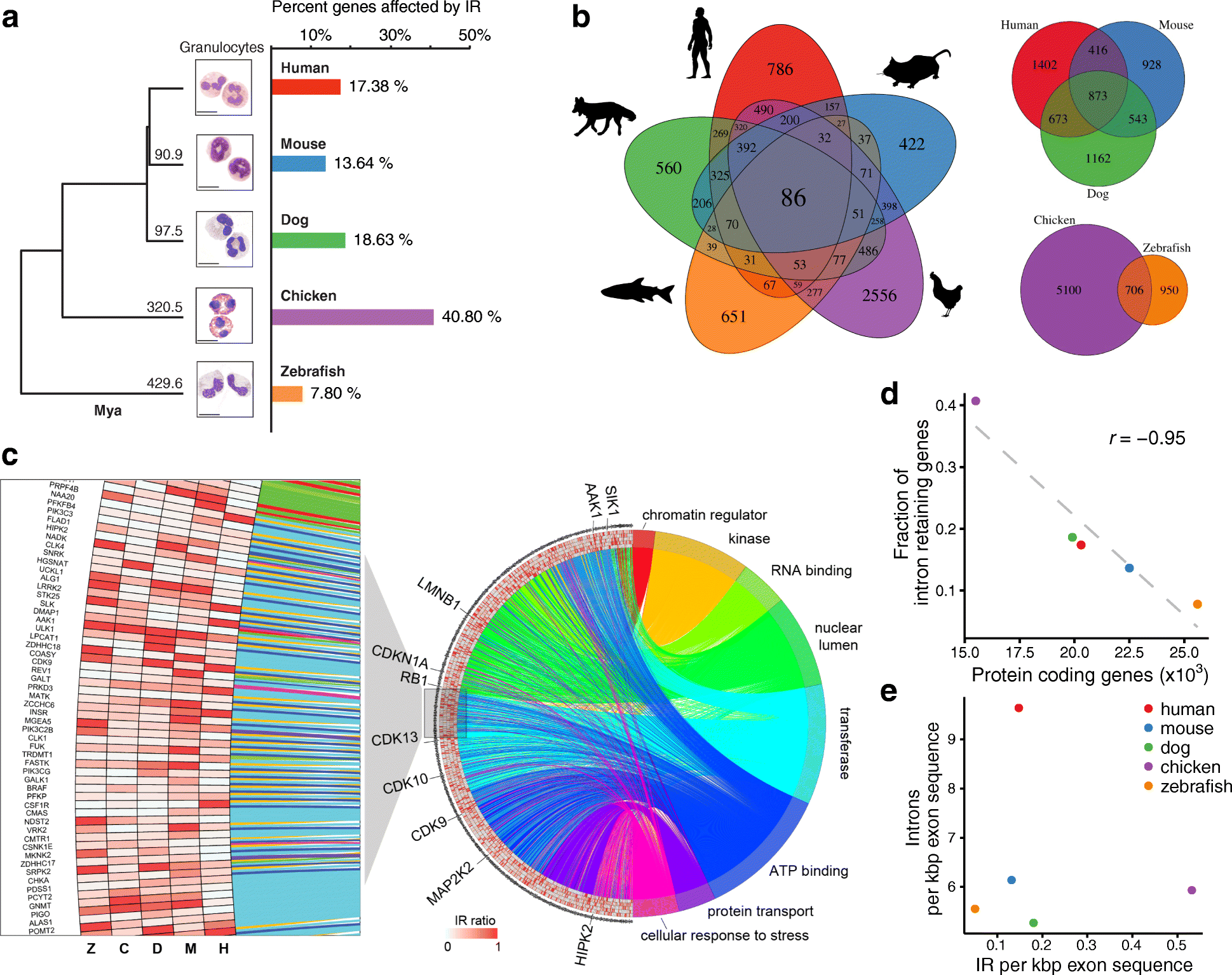
Background: While intron retention (IR) is now widely accepted as an important mechanism of mammalian gene expression control, it remains the least studied form of alternative splicing. To delineate conserved features of IR, we performed an exhaustive phylogenetic analysis in a highly purified and functionally defined cell type comprising neutrophilic granulocytes from five vertebrate species spanning 430 million years of evolution.
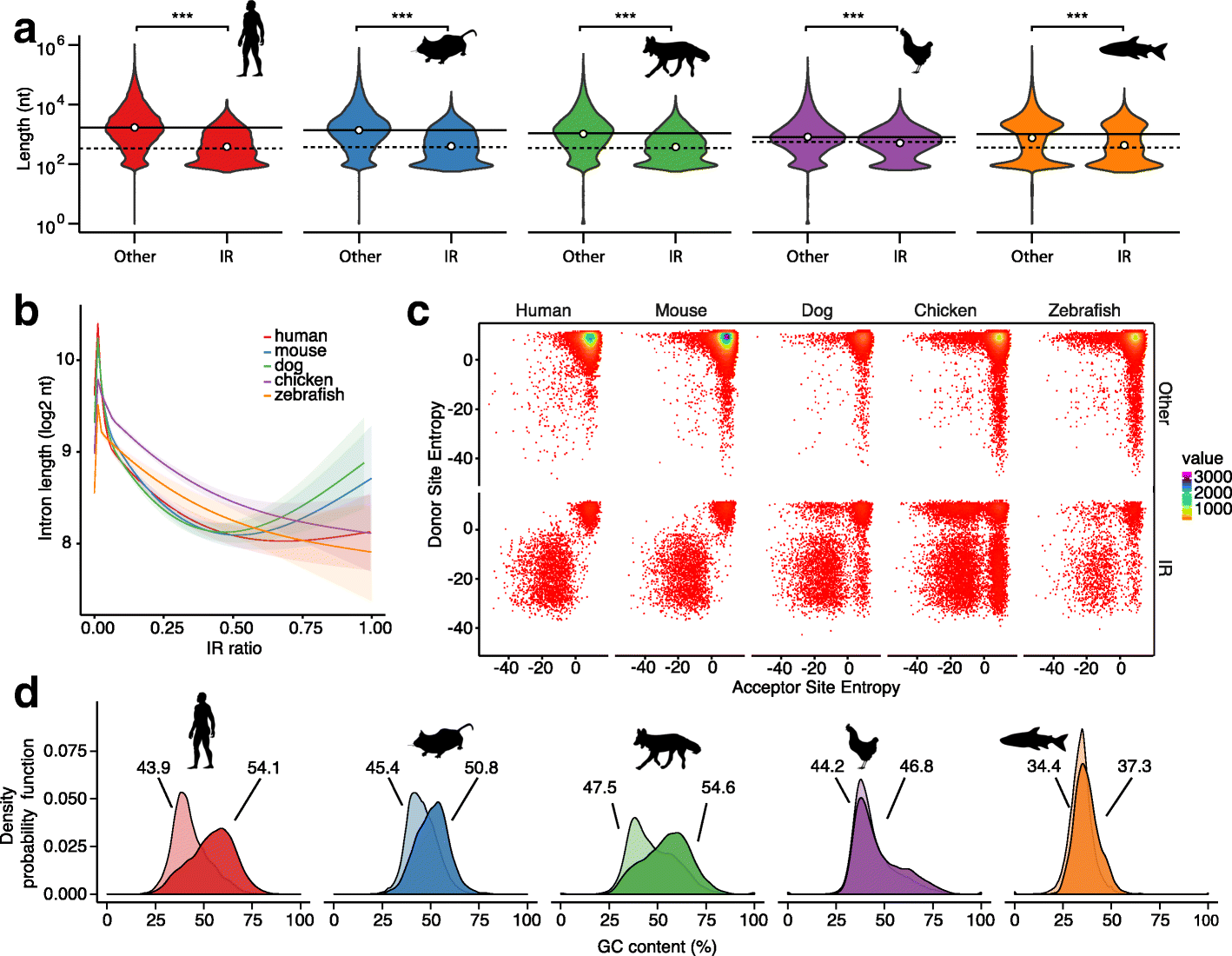
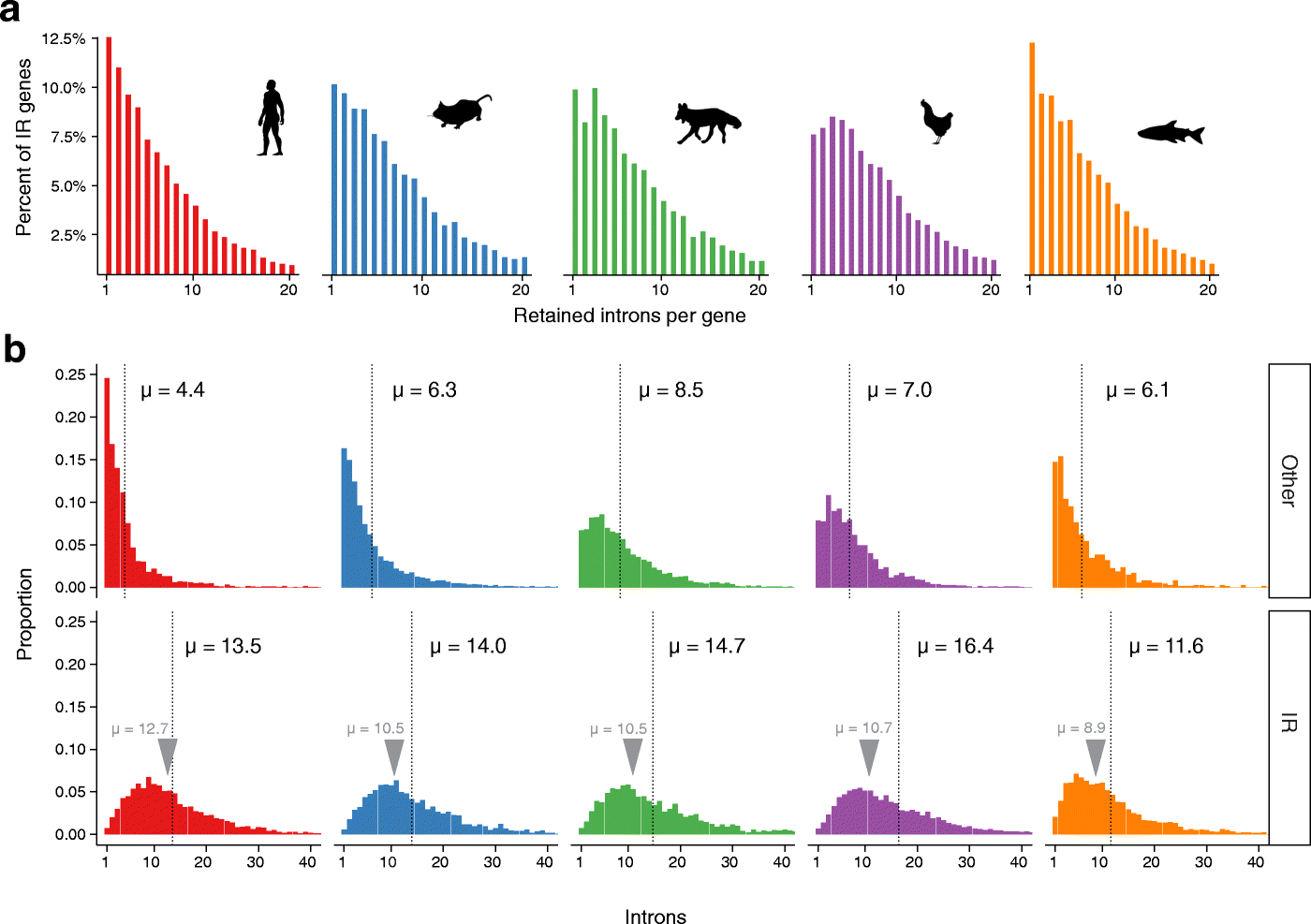
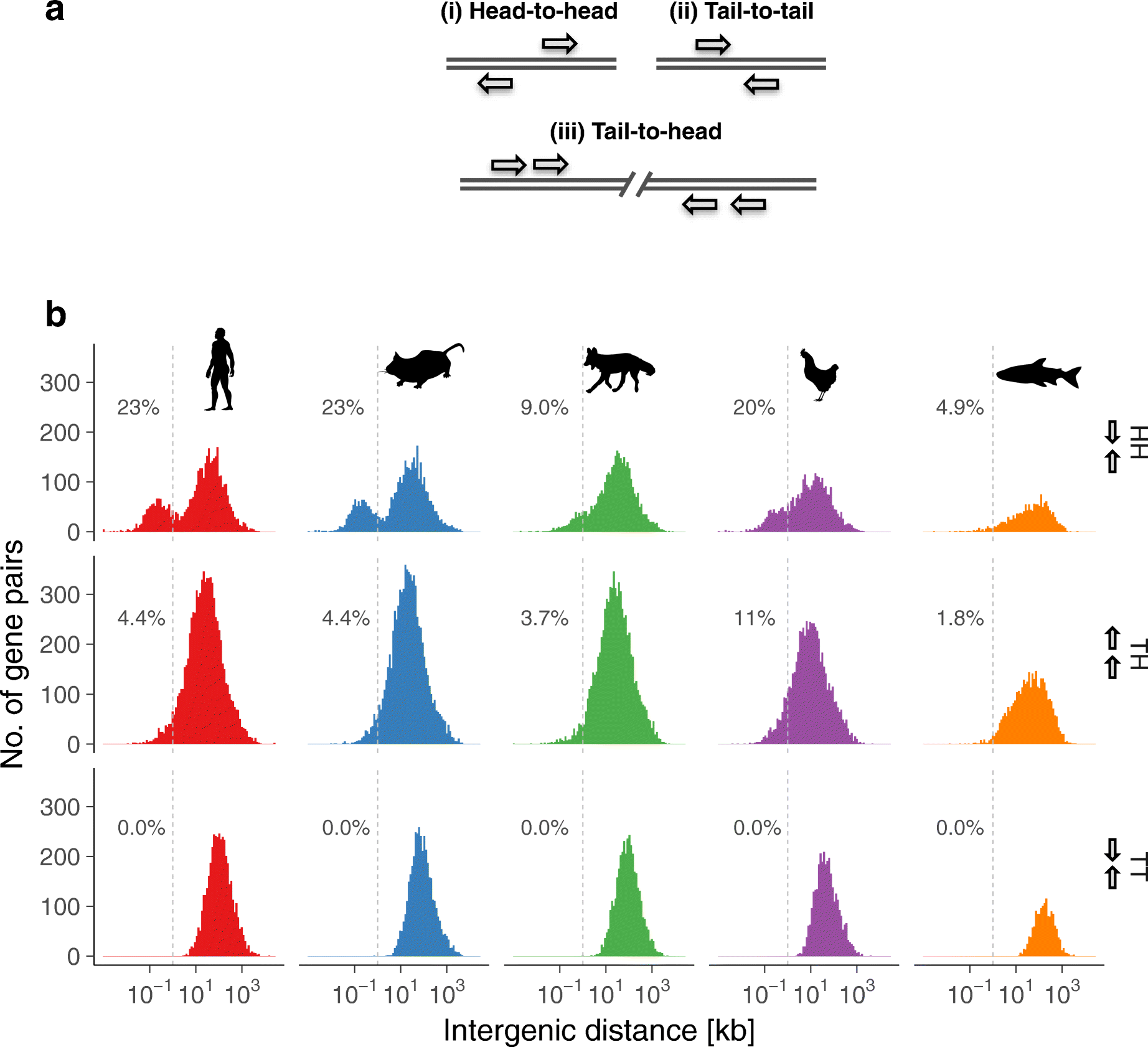
Results: Our RNA-sequencing-based analysis suggests that IR increases gene regulatory complexity, which is indicated by a strong anti-correlation between the number of genes affected by IR and the number of protein-coding genes in the genome of individual species. Our results confirm that IR affects many orthologous or functionally related genes in granulocytes. Further analysis uncovers new and unanticipated conserved characteristics of intron-retaining transcripts. We find that intron-retaining genes are transcriptionally co-regulated from bidirectional promoters. Intron-retaining genes have significantly longer 3' UTR sequences, with a corresponding increase in microRNA binding sites, some of which include highly conserved sequence motifs. This suggests that intron-retaining genes are highly regulated post-transcriptionally.
Conclusions: Our study provides unique insights concerning the role of IR as a robust and evolutionarily conserved mechanism of gene expression regulation. Our findings enhance our understanding of gene regulatory complexity by adding another contributor to evolutionary adaptation.
Keywords: Alternative splicing; Evolution; Gene regulation; Granulocytes; Intron retention; Transcriptomic complexity.
Conflict of interest statement
Ethics approval and consent to participate
The human whole blood sample was obtained with ethics approval from the Human Research Ethics Committee of the Royal Prince Alfred Hospital (HREC/08/RPAH/222). The human participant has given written informed consent. Experimental methods comply with the Helsinki Declaration. Ethics approval for the primary mouse granulocytes from bone marrow of male C57BL/6J mice (8–10 weeks) was obtained from RPAH AEC Protocol no. 2013/045. Dog peripheral blood samples were collected from Novartis Animal Health with ethics approval (Protocol no. YAR-13-001 and RPAH AEC Protocol no. 2013/045). Chicken peripheral blood was collected with ethics approval (UQ AEC no. SVS/365/13/ANNANDALE and RPAH AEC Protocol no. 2013/045). The zebrafish experiments were approved by the Monash University Animal Ethics Committee (protocol MAS-2010-18).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

