Intramuscular oxytocin versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery (LabOR trial): study protocol for a randomised controlled trial
- PMID: 29141679
- PMCID: PMC5688658
- DOI: 10.1186/s13063-017-2269-9
Intramuscular oxytocin versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery (LabOR trial): study protocol for a randomised controlled trial
Abstract
Background: Primary postpartum haemorrhage (PPH) is one of the leading causes of maternal morbidity and mortality worldwide. The most common cause of primary PPH is uterine atony. Atonic PPH rates are increasing in developed countries despite routine active management of the third stage of labour. In less-developed countries, primary PPH remains the leading cause of maternal death. Although the value of routine oxytocics in the third stage of labour has been well established, there is inconsistent practice in the choice of agent and route of administration. Oxytocin is the preferred agent because it has fewer side effects than other uterotonics with similar efficacy. It can be given intravenously or intramuscularly; however, to date, the most effective route of administering oxytocin has not been established.
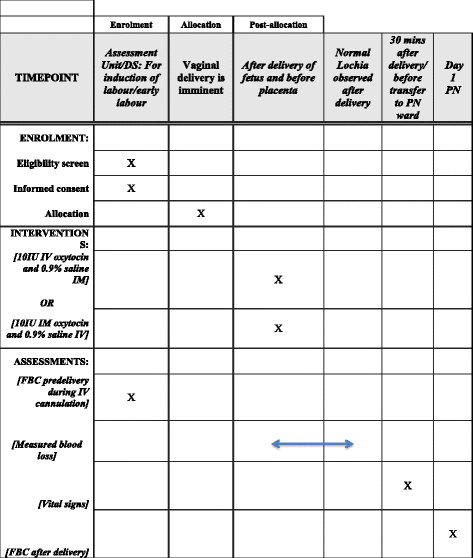
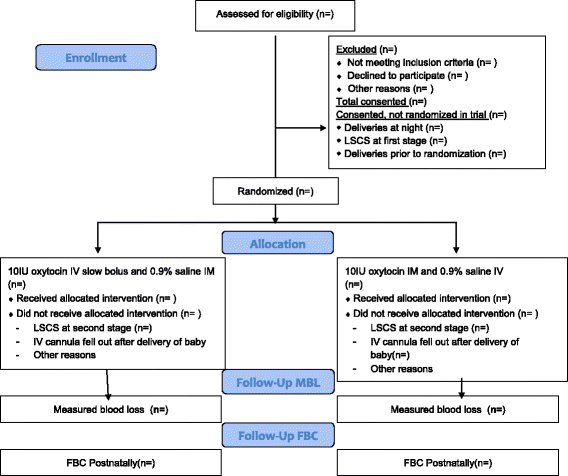
Methods/design: A double-blind randomised controlled trial is planned. The aim of the study is to compare the effects of an intramuscular bolus of oxytocin (10 IU in 1 mL) and placebo intravenous injection (1 mL 0.9% saline given slowly) with an intravenous bolus of oxytocin (10 IU in 1 mL given slowly over 1 min) and placebo intramuscular injection (1 mL 0.9% saline) at vaginal delivery. The study will recruit 1000 women at term (>36 weeks) with singleton pregnancies who are aiming for a vaginal delivery. The primary outcome will be PPH (measured blood loss ≥ 500 mL). A study involving 1000 women will have 80% power at the 5% two-sided alpha level, to detect differences in the proportion of patients with measured blood loss > 500 ml of 10% vs 5%.
Discussion: Given the increasing trends of atonic PPH it is both important and timely that we evaluate the most effective route of oxytocin administration for the management of the third stage of labour. To date, there has been limited research comparing the efficacy of intramuscular oxytocin vs intravenous oxytocin for the third stage of labour.
Trial registration: ISRCTN Registry, ISRCTN14718882 . Registered on 4 January 2016. Pilot commenced 12.12.2015; trial commenced 04.01.2016. The protocol (Ref 012012) was approved by the National Maternity Hospital Research Ethics Committee on 10.06.2015 and the Research Ethics Committee of the Coombe Women & Infants University Hospital (Ref 26-2015) on 09.12.2015.
Keywords: Active management third stage of labour; Intramuscular oxytocin; Intravenous oxytocin; Postpartum haemorrhage; Randomised controlled trial.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval from the National Maternity Hospital (Clinical Trials Committee: Protocol Ref 012012) was granted on 29 June 2015. The HPRA approved the trial application on 24 July 2015 (CT 0900/568/001Oxytocin). Ethics approval from the Coombe Women & Infants University Hospital (Ref 26-2015) was granted on 14 October 2015.
A research fellow/midwife will seek written informed consent if the following criteria are satisfied: (1) the woman meets the trial inclusion and exclusion criteria; (2) the midwife looking after the woman assesses her to be capable of providing informed consent; (3) the woman has adequate pain control; (4) the woman has not used systemic opiates in the last 4 h. At that point if the woman confirms interest in participation in the study she will be provided with a participant information leaflet, an opportunity to ask additional questions will be provided, and informed written consent will be completed.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Lewis G, Drife J, editors. Why mothers die 1997-1999. The fifth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: RCOG; 2001.
-
- Knight M, Callaghan WM, Berg C, Alexander S, Bouvier-Colle MH, Ford JB, et al. Trends in postpartum haemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009;9:55. doi: 10.1186/1471-2393-9-55. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical