Prostate-specific membrane antigen cleavage of vitamin B9 stimulates oncogenic signaling through metabotropic glutamate receptors
- PMID: 29141866
- PMCID: PMC5748857
- DOI: 10.1084/jem.20171052
Prostate-specific membrane antigen cleavage of vitamin B9 stimulates oncogenic signaling through metabotropic glutamate receptors
Erratum in
-
Correction: Prostate-specific membrane antigen cleavage of vitamin B9 stimulates oncogenic signaling through metabotropic glutamate receptors.J Exp Med. 2018 Jan 2;215(1):377. doi: 10.1084/jem.2017105211212017c. Epub 2017 Nov 28. J Exp Med. 2018. PMID: 29183990 Free PMC article. No abstract available.
Abstract
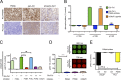
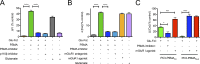
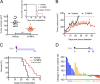
Prostate-specific membrane antigen (PSMA) or folate hydrolase 1 (FOLH1) is highly expressed on prostate cancer. Its expression correlates inversely with survival and increases with tumor grade. However, the biological role of PSMA has not been explored, and its role in prostate cancer remained elusive. Filling this gap, we demonstrate that in prostate cancer, PSMA initiates signaling upstream of PI3K through G protein-coupled receptors, specifically via the metabotropic glutamate receptor (mGluR). PSMA's carboxypeptidase activity releases glutamate from vitamin B9 and other glutamated substrates, which activate mGluR I. Activated mGluR I subsequently induces activation of phosphoinositide 3-kinase (PI3K) through phosphorylation of p110β independent of PTEN loss. The p110β isoform of PI3K plays a particularly important role in the pathogenesis of prostate cancer, but the origin of its activation was so far unknown. PSMA expression correlated with PI3K-Akt signaling in cells, animal models, and patients. We interrogated the activity of the PSMA-PI3K axis through positron emission tomography and magnetic resonance imaging. Inhibition of PSMA in preclinical models inhibited PI3K signaling and promoted tumor regression. Our data present a novel oncogenic signaling role of PSMA that can be exploited for therapy and interrogated with imaging.
© 2018 Kaittanis et al.
Figures




Comment in
-
PSMA brings new flavors to PI3K signaling: A role for glutamate in prostate cancer.J Exp Med. 2018 Jan 2;215(1):17-19. doi: 10.1084/jem.20172050. Epub 2017 Dec 8. J Exp Med. 2018. PMID: 29222106 Free PMC article.
References
-
- Carver B.S., Chapinski C., Wongvipat J., Hieronymus H., Chen Y., Chandarlapaty S., Arora V.K., Le C., Koutcher J., Scher H., et al. . 2011. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 19:575–586. 10.1016/j.ccr.2011.04.008 - DOI - PMC - PubMed
-
- Chang S.S., O’Keefe D.S., Bacich D.J., Reuter V.E., Heston W.D., and Gaudin P.B.. 1999. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin. Cancer Res. 5:2674–2681. - PubMed
-
- Colombatti M., Grasso S., Porzia A., Fracasso G., Scupoli M.T., Cingarlini S., Poffe O., Naim H.Y., Heine M., Tridente G., et al. . 2009. The prostate specific membrane antigen regulates the expression of IL-6 and CCL5 in prostate tumour cells by activating the MAPK pathways. PLoS One. 4:e4608 10.1371/journal.pone.0004608 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

