Igβ ubiquitination activates PI3K signals required for endosomal sorting
- PMID: 29141870
- PMCID: PMC5716028
- DOI: 10.1084/jem.20161868
Igβ ubiquitination activates PI3K signals required for endosomal sorting
Abstract
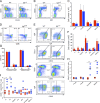
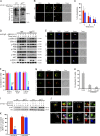
A wealth of in vitro data has demonstrated a central role for receptor ubiquitination in endocytic sorting. However, how receptor ubiquitination functions in vivo is poorly understood. Herein, we report that ablation of B cell antigen receptor ubiquitination in vivo uncouples the receptor from CD19 phosphorylation and phosphatidylinositol 3-kinase (PI3K) signals. These signals are necessary and sufficient for accumulating phosphatidylinositol (3,4,5)-trisphosphate (PIP3) on B cell receptor-containing early endosomes and proper sorting into the MHC class II antigen-presenting compartment (MIIC). Surprisingly, MIIC targeting is dispensable for T cell-dependent immunity. Rather, it is critical for activating endosomal toll-like receptors and antiviral humoral immunity. These findings demonstrate a novel mechanism of receptor endosomal signaling required for specific peripheral immune responses.
© 2017 Veselits et al.
Figures




Similar articles
-
IFITM3 functions as a PIP3 scaffold to amplify PI3K signalling in B cells.Nature. 2020 Dec;588(7838):491-497. doi: 10.1038/s41586-020-2884-6. Epub 2020 Nov 4. Nature. 2020. PMID: 33149299 Free PMC article.
-
Cutting edge: signals from the B lymphocyte antigen receptor regulate MHC class II containing late endosomes.J Immunol. 1998 Jun 1;160(11):5203-8. J Immunol. 1998. PMID: 9605114
-
Involvement of MIIC-like late endosomes in B cell receptor-mediated antigen processing in murine B cells.J Immunol. 1999 Jan 15;162(2):1150-5. J Immunol. 1999. PMID: 9916746
-
Receptors, subcellular compartments and the regulation of peripheral B cell responses: the illuminating state of anergy.Mol Immunol. 2011 Jun;48(11):1281-6. doi: 10.1016/j.molimm.2010.10.024. Epub 2010 Dec 7. Mol Immunol. 2011. PMID: 21144589 Free PMC article. Review.
-
The role of ubiquitylation in receptor endocytosis and endosomal sorting.J Cell Sci. 2012 Jan 15;125(Pt 2):265-75. doi: 10.1242/jcs.091280. J Cell Sci. 2012. PMID: 22357968 Review.
Cited by
-
Novel specialized cell state and spatial compartments within the germinal center.Nat Immunol. 2020 Jun;21(6):660-670. doi: 10.1038/s41590-020-0660-2. Epub 2020 Apr 27. Nat Immunol. 2020. PMID: 32341509 Free PMC article.
-
Itch regulation of innate and adaptive immune responses in mice and humans.J Leukoc Biol. 2020 Jul;108(1):353-362. doi: 10.1002/JLB.3MIR0320-272R. Epub 2020 Apr 30. J Leukoc Biol. 2020. PMID: 32356405 Free PMC article. Review.
-
Cbl and Cbl-b control the germinal center reaction by facilitating naive B cell antigen processing.J Exp Med. 2020 Sep 7;217(9):e20191537. doi: 10.1084/jem.20191537. J Exp Med. 2020. PMID: 32584413 Free PMC article.
-
B-Cell Receptor Signaling and Beyond: The Role of Igα (CD79a)/Igβ (CD79b) in Normal and Malignant B Cells.Int J Mol Sci. 2023 Dec 19;25(1):10. doi: 10.3390/ijms25010010. Int J Mol Sci. 2023. PMID: 38203179 Free PMC article. Review.
-
Regulation of B cell receptor-dependent NF-κB signaling by the tumor suppressor KLHL14.Proc Natl Acad Sci U S A. 2020 Mar 17;117(11):6092-6102. doi: 10.1073/pnas.1921187117. Epub 2020 Mar 3. Proc Natl Acad Sci U S A. 2020. PMID: 32127472 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

