Two Distinct Regulatory Mechanisms of Transcriptional Initiation in Response to Nutrient Signaling
- PMID: 29141908
- PMCID: PMC5753858
- DOI: 10.1534/genetics.117.300518
Two Distinct Regulatory Mechanisms of Transcriptional Initiation in Response to Nutrient Signaling
Abstract
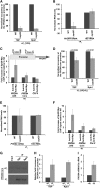
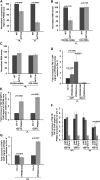
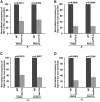
SAGA (Spt-Ada-Gcn5-Acetyltransferase) and TFIID (transcription factor IID) have been previously shown to facilitate the formation of the PIC (pre-initiation complex) at the promoters of two distinct sets of genes. Here, we demonstrate that TFIID and SAGA differentially participate in the stimulation of PIC formation (and hence transcriptional initiation) at the promoter of PHO84, a gene for the high-affinity inorganic phosphate (Pi) transporter for crucial cellular functions, in response to nutrient signaling. We show that transcriptional initiation of PHO84 occurs predominantly in a TFIID-dependent manner in the absence of Pi in the growth medium. Such TFIID dependency is mediated via the NuA4 (nucleosome acetyltransferase of H4) histone acetyltransferase (HAT). Intriguingly, transcriptional initiation of PHO84 also occurs in the presence of Pi in the growth medium, predominantly via the SAGA complex, but independently of NuA4 HAT. Thus, Pi in the growth medium switches transcriptional initiation of PHO84 from NuA4-TFIID to SAGA dependency. Further, we find that both NuA4-TFIID- and SAGA-dependent transcriptional initiations of PHO84 are facilitated by the 19S proteasome subcomplex or regulatory particle (RP) via enhanced recruitment of the coactivators SAGA and NuA4 HAT, which promote TFIID-independent and -dependent PIC formation for transcriptional initiation, respectively. NuA4 HAT does not regulate activator binding to PHO84, but rather facilitates PIC formation for transcriptional initiation in the absence of Pi in the growth medium. On the other hand, SAGA promotes activator recruitment to PHO84 for transcriptional initiation in the growth medium containing Pi. Collectively, our results demonstrate two distinct stimulatory pathways for PIC formation (and hence transcriptional initiation) at PHO84 by TFIID, SAGA, NuA4, and 19S RP in the presence and absence of an essential nutrient, Pi, in the growth media, thus providing new regulatory mechanisms of transcriptional initiation in response to nutrient signaling.
Keywords: 19S RP; NuA4; SAGA; TFIID; transcription.
Copyright © 2018 by the Genetics Society of America.
Figures




References
-
- Bevington A., Kemp G. J., Graham R., Russell G., 1992. Phosphate-sensitive enzymes: possible molecular basis for cellular disorders of phosphate metabolism. Clin. Chem. Enzym. Comms. 4: 235–257.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

