Constraints on lateral gene transfer in promoting fimbrial usher protein diversity and function
- PMID: 29142104
- PMCID: PMC5717340
- DOI: 10.1098/rsob.170144
Constraints on lateral gene transfer in promoting fimbrial usher protein diversity and function
Abstract
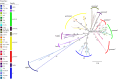
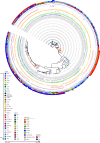
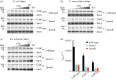
Fimbriae are long, adhesive structures widespread throughout members of the family Enterobacteriaceae. They are multimeric extrusions, which are moved out of the bacterial cell through an integral outer membrane protein called usher. The complex folding mechanics of the usher protein were recently revealed to be catalysed by the membrane-embedded translocation and assembly module (TAM). Here, we examine the diversity of usher proteins across a wide range of extraintestinal (ExPEC) and enteropathogenic (EPEC) Escherichia coli, and further focus on a so far undescribed chaperone-usher system, with this usher referred to as UshC. The fimbrial system containing UshC is distributed across a discrete set of EPEC types, including model strains like E2348/67, as well as ExPEC ST131, currently the most prominent multi-drug-resistant uropathogenic E. coli strain worldwide. Deletion of the TAM from a naive strain of E. coli results in a drastic time delay in folding of UshC, which can be observed for a protein from EPEC as well as for two introduced proteins from related organisms, Yersinia and Enterobacter We suggest that this models why the TAM machinery is essential for efficient folding of proteins acquired via lateral gene transfer.
Keywords: fimbriae; outer membrane; translocation and assembly module.
© 2017 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures



Similar articles
-
The small molecule nitazoxanide selectively disrupts BAM-mediated folding of the outer membrane usher protein.J Biol Chem. 2019 Sep 27;294(39):14357-14369. doi: 10.1074/jbc.RA119.009616. Epub 2019 Aug 7. J Biol Chem. 2019. PMID: 31391254 Free PMC article.
-
Chaperone-usher fimbriae of Escherichia coli.PLoS One. 2013;8(1):e52835. doi: 10.1371/journal.pone.0052835. Epub 2013 Jan 30. PLoS One. 2013. PMID: 23382825 Free PMC article.
-
Nitazoxanide Inhibits Pilus Biogenesis by Interfering with Folding of the Usher Protein in the Outer Membrane.Antimicrob Agents Chemother. 2016 Mar 25;60(4):2028-38. doi: 10.1128/AAC.02221-15. Print 2016 Apr. Antimicrob Agents Chemother. 2016. PMID: 26824945 Free PMC article.
-
Molecular and structural aspects of fimbriae biosynthesis and assembly in Escherichia coli.FEMS Microbiol Rev. 1996 Oct;19(1):25-52. doi: 10.1111/j.1574-6976.1996.tb00252.x. FEMS Microbiol Rev. 1996. PMID: 8916554 Review.
-
The TAM: A Translocation and Assembly Module of the β-Barrel Assembly Machinery in Bacterial Outer Membranes.EcoSal Plus. 2019 Feb;8(2):10.1128/ecosalplus.ESP-0036-2018. doi: 10.1128/ecosalplus.ESP-0036-2018. EcoSal Plus. 2019. PMID: 30816086 Free PMC article. Review.
Cited by
-
The TAM, a Translocation and Assembly Module for protein assembly and potential conduit for phospholipid transfer.EMBO Rep. 2024 Apr;25(4):1711-1720. doi: 10.1038/s44319-024-00111-y. Epub 2024 Mar 11. EMBO Rep. 2024. PMID: 38467907 Free PMC article. Review.
-
Configuration of gut bacterial community profile and their potential functionality in the digestive tract of the wild and cultivated Indonesian shortfin elver-phase eels (Anguilla bicolor bicolor McClelland, 1844).3 Biotech. 2023 May;13(5):153. doi: 10.1007/s13205-023-03561-8. Epub 2023 Apr 29. 3 Biotech. 2023. PMID: 37131968 Free PMC article.
-
Recent Advances in Our Understanding of the Diversity and Roles of Chaperone-Usher Fimbriae in Facilitating Salmonella Host and Tissue Tropism.Front Cell Infect Microbiol. 2021 Feb 3;10:628043. doi: 10.3389/fcimb.2020.628043. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33614531 Free PMC article. Review.
-
The translocation assembly module (TAM) catalyzes the assembly of bacterial outer membrane proteins in vitro.bioRxiv [Preprint]. 2024 Jun 20:2024.06.20.599893. doi: 10.1101/2024.06.20.599893. bioRxiv. 2024. Update in: Nat Commun. 2024 Aug 23;15(1):7246. doi: 10.1038/s41467-024-51628-8. PMID: 39372782 Free PMC article. Updated. Preprint.
-
Classical chaperone-usher (CU) adhesive fimbriome: uropathogenic Escherichia coli (UPEC) and urinary tract infections (UTIs).Folia Microbiol (Praha). 2020 Feb;65(1):45-65. doi: 10.1007/s12223-019-00719-x. Epub 2019 Jun 5. Folia Microbiol (Praha). 2020. PMID: 31165977 Review.
References
-
- Ochman H, Lawrence JG, Groisman EA. 2000. Lateral gene transfer and the nature of bacterial innovation. Nature 405, 299–304. (doi:10.1038/35012500) - DOI - PubMed
-
- Thomas CM, Nielsen KM. 2005. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 3, 711–721. (doi:10.1038/nrmicro1234) - DOI - PubMed
-
- Boto L. 2010. Horizontal gene transfer in evolution: facts and challenges. Proc. R. Soc. B 277, 819–827. (doi:10.1098/rspb.2009.1679) - DOI - PMC - PubMed
-
- Leimbach A, Hacker J, Dobrindt U. 2013. E. coli as an all-rounder: the thin line between commensalism and pathogenicity. Curr. Top. Microbiol. Immunol. 358, 3–32. (doi:10.1007/82_2012_303) - DOI - PubMed
-
- Roberts AP, Kreth J. 2014. The impact of horizontal gene transfer on the adaptive ability of the human oral microbiome. Front. Cell. Infect. Microbiol. 4, 124 (doi:10.3389/fcimb.2014.00124) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

