Entry of Human Coronavirus NL63 into the Cell
- PMID: 29142129
- PMCID: PMC5774871
- DOI: 10.1128/JVI.01933-17
Entry of Human Coronavirus NL63 into the Cell
Abstract
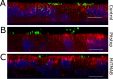
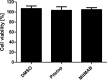
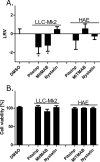
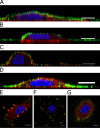
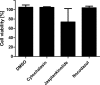
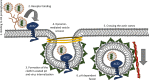
The first steps of human coronavirus NL63 (HCoV-NL63) infection were previously described. The virus binds to target cells by use of heparan sulfate proteoglycans and interacts with the ACE2 protein. Subsequent events, including virus internalization and trafficking, remain to be elucidated. In this study, we mapped the process of HCoV-NL63 entry into the LLC-Mk2 cell line and ex vivo three-dimensional (3D) tracheobronchial tissue. Using a variety of techniques, we have shown that HCoV-NL63 virions require endocytosis for successful entry into the LLC-MK2 cells, and interaction between the virus and the ACE2 molecule triggers recruitment of clathrin. Subsequent vesicle scission by dynamin results in virus internalization, and the newly formed vesicle passes the actin cortex, which requires active cytoskeleton rearrangement. Finally, acidification of the endosomal microenvironment is required for successful fusion and release of the viral genome into the cytoplasm. For 3D tracheobronchial tissue cultures, we also observed that the virus enters the cell by clathrin-mediated endocytosis, but we obtained results suggesting that this pathway may be bypassed.IMPORTANCE Available data on coronavirus entry frequently originate from studies employing immortalized cell lines or undifferentiated cells. Here, using the most advanced 3D tissue culture system mimicking the epithelium of conductive airways, we systematically mapped HCoV-NL63 entry into susceptible cells. The data obtained allow for a better understanding of the infection process and may support development of novel treatment strategies.
Keywords: Coronaviridae; HCoV-NL63; clathrin; coronavirus; endocytosis; entry; infection; internalization.
Copyright © 2018 American Society for Microbiology.
Figures
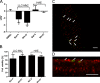


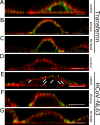
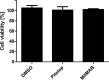
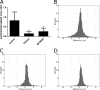


References
-
- Mayer K, Nellessen C, Hahn-Ast C, Schumacher M, Pietzonka S, Eis-Hübinger AM, Drosten C, Brossart P, Wolf D. 2016. Fatal outcome of human coronavirus NL63 infection despite successful viral elimination by IFN-alpha in a patient with newly diagnosed ALL. Eur J Haematol 97:208–210. doi:10.1111/ejh.12744. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

