Identification of a Small Molecule That Compromises the Structural Integrity of Viroplasms and Rotavirus Double-Layered Particles
- PMID: 29142132
- PMCID: PMC5774888
- DOI: 10.1128/JVI.01943-17
Identification of a Small Molecule That Compromises the Structural Integrity of Viroplasms and Rotavirus Double-Layered Particles
Abstract
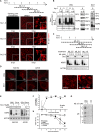
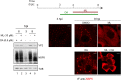
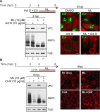
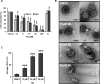
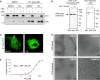
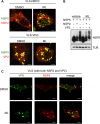
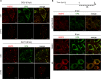
Despite the availability of two attenuated vaccines, rotavirus (RV) gastroenteritis remains an important cause of mortality among children in developing countries, causing about 215,000 infant deaths annually. Currently, there are no specific antiviral therapies available. RV is a nonenveloped virus with a segmented double-stranded RNA genome. Viral genome replication and assembly of transcriptionally active double-layered particles (DLPs) take place in cytoplasmic viral structures called viroplasms. In this study, we describe strong impairment of the early stages of RV replication induced by a small molecule known as an RNA polymerase III inhibitor, ML-60218 (ML). This compound was found to disrupt already assembled viroplasms and to hamper the formation of new ones without the need for de novo transcription of cellular RNAs. This phenotype was correlated with a reduction in accumulated viral proteins and newly made viral genome segments, disappearance of the hyperphosphorylated isoforms of the viroplasm-resident protein NSP5, and inhibition of infectious progeny virus production. In in vitro transcription assays with purified DLPs, ML showed dose-dependent inhibitory activity, indicating the viral nature of its target. ML was found to interfere with the formation of higher-order structures of VP6, the protein forming the DLP outer layer, without compromising its ability to trimerize. Electron microscopy of ML-treated DLPs showed dose-dependent structural damage. Our data suggest that interactions between VP6 trimers are essential, not only for DLP stability, but also for the structural integrity of viroplasms in infected cells.IMPORTANCE Rotavirus gastroenteritis is responsible for a large number of infant deaths in developing countries. Unfortunately, in the countries where effective vaccines are urgently needed, the efficacy of the available vaccines is particularly low. Therefore, the development of antivirals is an important goal, as they might complement the available vaccines or represent an alternative option. Moreover, they may be decisive in fighting the acute phase of infection. This work describes the inhibitory effect on rotavirus replication of a small molecule initially reported as an RNA polymerase III inhibitor. The molecule is the first chemical compound identified that is able to disrupt viroplasms, the viral replication machinery, and to compromise the stability of DLPs by targeting the viral protein VP6. This molecule thus represents a starting point in the development of more potent and less cytotoxic compounds against rotavirus infection.
Keywords: DLP; ML-60218; RNA polymerase III; VP6; antivirals; drug; inhibitor; rotavirus; viroplasms.
Copyright © 2018 American Society for Microbiology.
Figures

Similar articles
-
Recombinant Rotaviruses Rescued by Reverse Genetics Reveal the Role of NSP5 Hyperphosphorylation in the Assembly of Viral Factories.J Virol. 2019 Dec 12;94(1):e01110-19. doi: 10.1128/JVI.01110-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31619556 Free PMC article.
-
The recruitment of TRiC chaperonin in rotavirus viroplasms correlates with virus replication.mBio. 2024 Apr 10;15(4):e0049924. doi: 10.1128/mbio.00499-24. Epub 2024 Mar 12. mBio. 2024. PMID: 38470055 Free PMC article.
-
Conserved Rotavirus NSP5 and VP2 Domains Interact and Affect Viroplasm.J Virol. 2020 Mar 17;94(7):e01965-19. doi: 10.1128/JVI.01965-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31915278 Free PMC article.
-
The Role of the Host Cytoskeleton in the Formation and Dynamics of Rotavirus Viroplasms.Viruses. 2024 Apr 25;16(5):668. doi: 10.3390/v16050668. Viruses. 2024. PMID: 38793550 Free PMC article. Review.
-
Sneaking into the viral safe-houses: Implications of host components in regulating integrity and dynamics of rotaviral replication factories.Front Cell Infect Microbiol. 2022 Sep 14;12:977799. doi: 10.3389/fcimb.2022.977799. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36189370 Free PMC article. Review.
Cited by
-
Recombinant Rotaviruses Rescued by Reverse Genetics Reveal the Role of NSP5 Hyperphosphorylation in the Assembly of Viral Factories.J Virol. 2019 Dec 12;94(1):e01110-19. doi: 10.1128/JVI.01110-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31619556 Free PMC article.
-
Advances in the Development of Antiviral Compounds for Rotavirus Infections.mBio. 2021 May 11;12(3):e00111-21. doi: 10.1128/mBio.00111-21. mBio. 2021. PMID: 33975930 Free PMC article. Review.
-
Principles of RNA recruitment to viral ribonucleoprotein condensates in a segmented dsRNA virus.Elife. 2023 Jan 26;12:e68670. doi: 10.7554/eLife.68670. Elife. 2023. PMID: 36700549 Free PMC article.
-
Rotaviruses: From Pathogenesis to Disease Control-A Critical Review.Viruses. 2022 Apr 22;14(5):875. doi: 10.3390/v14050875. Viruses. 2022. PMID: 35632617 Free PMC article. Review.
-
Rotavirus Spike Protein VP4 Mediates Viroplasm Assembly by Association to Actin Filaments.J Virol. 2022 Sep 14;96(17):e0107422. doi: 10.1128/jvi.01074-22. Epub 2022 Aug 8. J Virol. 2022. PMID: 35938869 Free PMC article.
References
-
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization-Coordinated Global Rotavirus Surveillance Network . 2016. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000-2013. Clin Infect Dis 62(Suppl 2):S96–S105. doi:10.1093/cid/civ1013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

