Mild heat induces a distinct "eustress" response in Chinese Hamster Ovary cells but does not induce heat shock protein synthesis
- PMID: 29142280
- PMCID: PMC5688065
- DOI: 10.1038/s41598-017-15821-8
Mild heat induces a distinct "eustress" response in Chinese Hamster Ovary cells but does not induce heat shock protein synthesis
Abstract
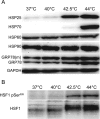
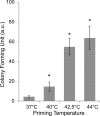
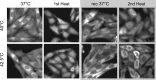
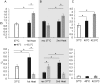
The current research on cellular heat stress management focuses on the roles of heat shock proteins (HSPs) and the proteostasis network under severe stress conditions. The mild, fever-type stress and the maintenance of membrane homeostasis are less well understood. Herein, we characterized the acute effect of mild, fever-range heat shock on membrane organization, and HSP synthesis and localization in two mammalian cell lines, to delineate the role of membranes in the sensing and adaptation to heat. A multidisciplinary approach combining ultrasensitive fluorescence microscopy and lipidomics revealed the molecular details of novel cellular "eustress", when cells adapt to mild heat by maintaining membrane homeostasis, activating lipid remodeling, and redistributing chaperone proteins. Notably, this leads to acquired thermotolerance in the complete absence of the induction of HSPs. At higher temperatures, additional defense mechanisms are activated, including elevated expression of molecular chaperones, contributing to an extended stress memory and acquired thermotolerance.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Selye, H. Stress without Distress. in Psychopathology of Human Adaptation 137–146 (Springer US, 1976). 10.1007/978-1-4684-2238-2_9
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

