Hypercholesterolemia Enhances T Cell Receptor Signaling and Increases the Regulatory T Cell Population
- PMID: 29142309
- PMCID: PMC5688061
- DOI: 10.1038/s41598-017-15546-8
Hypercholesterolemia Enhances T Cell Receptor Signaling and Increases the Regulatory T Cell Population
Abstract
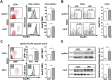
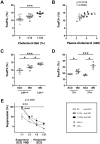
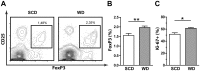
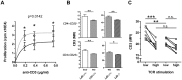
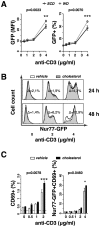
Hypercholesterolemia promotes the inflammation against lipoproteins in atherosclerosis. Development of atherosclerosis is affected by the balance between pro-inflammatory effector T cells and anti-inflammatory regulatory T (Treg) cells. However, phenotype and function of T cell subpopulations in hypercholesterolemia remain to be investigated. Here, we found that cholesterol-containing diet increased the expression of the Treg cell lineage-defining transcription factor FoxP3 among thymocytes and splenocytes. Hypercholesterolemia elevated the FoxP3 expression level and population size of peripheral Treg cells, but did not prevent enhanced proliferation of stimulated T cells. Moreover, cholesterol supplementation in diet as well as in cell culture medium promoted T cell antigen receptor (TCR) signaling in CD4+ T cells. Our results demonstrate that hypercholesterolemia enhances TCR stimulation, Treg cell development as well as T cell proliferation. Thus, our findings may help to understand why hypercholesterolemia correlates with altered CD4+ T cell responses.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

