Sodium channel NaV1.3 is important for enterochromaffin cell excitability and serotonin release
- PMID: 29142310
- PMCID: PMC5688111
- DOI: 10.1038/s41598-017-15834-3
Sodium channel NaV1.3 is important for enterochromaffin cell excitability and serotonin release
Abstract
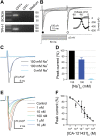
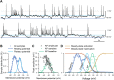
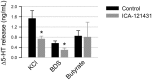
In the gastrointestinal (GI) epithelium, enterochromaffin (EC) cells are enteroendocrine cells responsible for producing >90% of the body's serotonin (5-hydroxytryptamine, 5-HT). However, the molecular mechanisms of EC cell function are poorly understood. Here, we found that EC cells in mouse primary cultures fired spontaneous bursts of action potentials. We examined the repertoire of voltage-gated sodium channels (NaV) in fluorescence-sorted mouse EC cells and found that Scn3a was highly expressed. Scn3a-encoded NaV1.3 was specifically and densely expressed at the basal side of both human and mouse EC cells. Using electrophysiology, we found that EC cells expressed robust NaV1.3 currents, as determined by their biophysical and pharmacologic properties. NaV1.3 was not only critical for generating action potentials in EC cells, but it was also important for regulating 5-HT release by these cells. Therefore, EC cells use Scn3a-encoded voltage-gated sodium channel NaV1.3 for electrical excitability and 5-HT release. NaV1.3-dependent electrical excitability and its contribution to 5-HT release is a novel mechanism of EC cell function.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
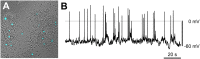



References
-
- Keating DJ, Spencer NJ. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology. 2010;138(659–670):670 e651–652. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

