Design of Cultured Neuron Networks in vitro with Predefined Connectivity Using Asymmetric Microfluidic Channels
- PMID: 29142321
- PMCID: PMC5688062
- DOI: 10.1038/s41598-017-15506-2
Design of Cultured Neuron Networks in vitro with Predefined Connectivity Using Asymmetric Microfluidic Channels
Abstract
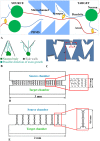
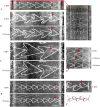
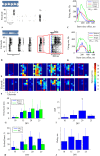
The architecture of neuron connectivity in brain networks is one of the basic mechanisms by which to organize and sustain a particular function of the brain circuitry. There are areas of the brain composed of well-organized layers of neurons connected by unidirectional synaptic connections (e.g., cortex, hippocampus). Re-engineering of the neural circuits with such a heterogeneous network structure in culture may uncover basic mechanisms of emergent information functions of these circuits. In this study, we present such a model designed with two subpopulations of primary hippocampal neurons (E18) with directed connectivity grown in a microfluidic device with asymmetric channels. We analysed and compared neurite growth in the microchannels with various shapes that promoted growth dominantly in one direction. We found an optimal geometric shape features of the microchannels in which the axons coupled two chambers with the neurons. The axons grew in the promoted direction and formed predefined connections during the first 6 days in vitro (DIV). The microfluidic devices were coupled with microelectrode arrays (MEAs) to confirm unidirectional spiking pattern propagation through the microchannels between two compartments. We found that, during culture development, the defined morphological and functional connectivity formed and was maintained for up to 25 DIV.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

