Are Polyunsaturated Fatty Acid Metabolites, the Protective Effect of 4-hydroxytyrosol on Human Red Blood Cell Membranes and Oxidative Damage (4-hydroxyalkenals) Compatible in Hypertriglyceridemic Patients?
- PMID: 29142415
- PMCID: PMC5669098
- DOI: 10.4103/pm.pm_483_15
Are Polyunsaturated Fatty Acid Metabolites, the Protective Effect of 4-hydroxytyrosol on Human Red Blood Cell Membranes and Oxidative Damage (4-hydroxyalkenals) Compatible in Hypertriglyceridemic Patients?
Abstract
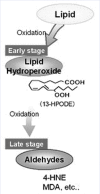
Background: Increased levels of malondialdehyde (MDA) and 4-hydroxynonenal (HNE) are demonstrated in plasma of uremic patients. A study showed that the comparison of erythrocytes of healthy and diseased patients (obese, hypertensive, and Type 2 diabetics) with age is associated to a disturbed oxidant/antioxidant balance when obesity is associated with hypertension. 4-hydroxytyrosol is shown to significantly protect red blood cells (RBCs) from oxidative damage (4-HNE). In literature, there are partial discussions on the role of lipids and their oxidation products. The products of degradation of membrane proteins are observed as self-consisting products without interrelations with membrane lipids.
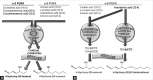
Objective: The aim of this study is to evaluate the role of polyunsaturated fatty acid (PUFA) metabolites on oxidative damage (4-hydroxy-alkenals) in RBCs of hypertriglyceridemic patients after membrane treatment with 4-hydroxytyrosol.
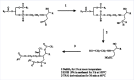
Materials and methods: The authors optimize the isolation of RBC ghosts and spectrophotometric method to measure free 4-hydroxyalkenals in human RBC membranes and investigated the effect on oxidative damage in human erythrocyte membranes and in vitro 4-hydroxytyrosol treatment to evaluate the membrane lipids reducible by this phenol.
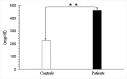
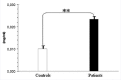
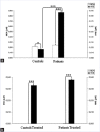
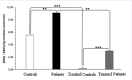
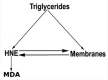
Results: Plasma triglyceride levels in patients are clearly higher than in controls. Moreover, total membrane proteins data are similar to previous described. The normalized alkenals levels are significantly enhanced in hyperlipemic patients in comparison to normoglyceridemic controls. After the 4-hydroxytyrosol action, lipid metabolites substantially decrease. The ratio of oxidized lipids (MDA + HNE) and membrane proteins data are similar to previously described ones.
Conclusion: According to experimental data, the accumulation of the alkenals in RBC membrane could be produced either by partial PUFA oxidation contained in glycerides and plasma glycerides and by glycerides into plasma membrane recycled RBC.
Summary: Hypertriglyceridemia induces oxidative stress in human red blood cell (RBC) membranesOxidative stress causes increased plasma membrane total protein concentration and hydroxynonenal and malondialdehyde levelsThe authors optimize the isolation of RBC ghosts and spectrophotometric method to measure free 4-hydroxyalkenals in human RBC membranesAfter the reduction with 4-hydroxytyrosol, oxidized lipid concentration significantly decrease. Abbreviations used: RBC: Red blood cell; MDA: Malondialdehyde; HNE\HAE: 4-hydroxyalkenals; LPO: Lipid peroxidation; ROS: Reactive oxygen species; ORAC: Oxygen Radical Absorbance Capacity.
Keywords: 4-hydroxyalkenals; 4-hydroxytyrosol; hypertriglyceridemia; lipid peroxidation; malondialdehyde; oxidative stress.
Conflict of interest statement
There are no conflicts of interest.
Figures


Similar articles
-
Oxidative stress in families of type 1 diabetic patients.Diabetes Care. 2000 Aug;23(8):1182-6. doi: 10.2337/diacare.23.8.1182. Diabetes Care. 2000. PMID: 10937519
-
Red blood cell susceptibility to lipid peroxidation, membrane lipid composition, and antioxidant enzymes in continuous ambulatory peritoneal dialysis patients.Perit Dial Int. 1992;12(2):205-10. Perit Dial Int. 1992. PMID: 1586681
-
Metabolomic Analysis and Antioxidant Effect of Amla (Emblica officinalis) Extract in Preventing Oxidative Stress-Induced Red Cell Damage and Plasma Protein Alterations: An In Vitro Study.J Med Food. 2018 Jan;21(1):81-89. doi: 10.1089/jmf.2017.3942. Epub 2017 Oct 24. J Med Food. 2018. PMID: 29064307
-
Hormetic and regulatory effects of lipid peroxidation mediators in pancreatic beta cells.Mol Aspects Med. 2016 Jun;49:49-77. doi: 10.1016/j.mam.2016.03.001. Epub 2016 Mar 21. Mol Aspects Med. 2016. PMID: 27012748 Review.
-
Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes.Free Radic Biol Med. 2013 Dec;65:978-987. doi: 10.1016/j.freeradbiomed.2013.08.163. Epub 2013 Aug 20. Free Radic Biol Med. 2013. PMID: 23973638 Review.
Cited by
-
Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging.Oncotarget. 2018 Mar 30;9(24):17181-17198. doi: 10.18632/oncotarget.24729. eCollection 2018 Mar 30. Oncotarget. 2018. PMID: 29682215 Free PMC article. Review.
-
Dietary Thiols: A Potential Supporting Strategy against Oxidative Stress in Heart Failure and Muscular Damage during Sports Activity.Int J Environ Res Public Health. 2020 Dec 16;17(24):9424. doi: 10.3390/ijerph17249424. Int J Environ Res Public Health. 2020. PMID: 33339141 Free PMC article. Review.
-
Can Medicinal Plants and Bioactive Compounds Combat Lipid Peroxidation Product 4-HNE-Induced Deleterious Effects?Biomolecules. 2020 Jan 16;10(1):146. doi: 10.3390/biom10010146. Biomolecules. 2020. PMID: 31963301 Free PMC article. Review.
References
-
- Sommerburg O, Grune T, Hampl H, Riedel E, van Kuijk FJ, Ehrich JH, et al. Does long-term treatment of renal anaemia with recombinant erythropoietin influence oxidative stress in haemodialysed patients? Nephrol Dial Transplant. 1998;13:2583–7. - PubMed
-
- Breusing N, Grune T, Andrisic L, Atalay M, Bartosz G, Biasi F, et al. An inter-laboratory validation of methods of lipid peroxidation measurement in UVA-treated human plasma samples. Free Radic Res. 2010;44:1203–15. - PubMed
-
- Paiva-Martins F, Fernandes J, Rocha S, Nascimento H, Vitorino R, Amado F, et al. Effects of olive oil polyphenols on erythrocyte oxidative damage. Mol Nutr Food Res. 2009;53:609–16. - PubMed
-
- Bali EB, Ergin V, Rackova L, Bayraktar O, Küçükboyaci N, Karasu Ç. Olive leaf extracts protect cardiomyocytes against 4-hydroxynonenal-induced toxicity in vitro: Comparison with oleuropein, hydroxytyrosol, and quercetin. Planta Med. 2014;80:984–92. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
