Prostaglandin E1 protects hepatocytes against endoplasmic reticulum stress-induced apoptosis via protein kinase A-dependent induction of glucose-regulated protein 78 expression
- PMID: 29142472
- PMCID: PMC5677201
- DOI: 10.3748/wjg.v23.i40.7253
Prostaglandin E1 protects hepatocytes against endoplasmic reticulum stress-induced apoptosis via protein kinase A-dependent induction of glucose-regulated protein 78 expression
Abstract
Aim: To investigate the protective effect of prostaglandin E1 (PGE1) against endoplasmic reticulum (ER) stress-induced hepatocyte apoptosis, and to explore its underlying mechanisms.
Methods: Thapsigargin (TG) was used to induce ER stress in the human hepatic cell line L02 and hepatocarcinoma-derived cell line HepG2. To evaluate the effects of PGE1 on TG-induced apoptosis, PGE1 was used an hour prior to TG treatment. Activation of unfolded protein response signaling pathways were detected by western blotting and quantitative real-time RT-PCR. Apoptotic index and cell viability of L02 cells and HepG2 cells were determined with flow cytometry and MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay.
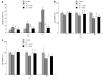
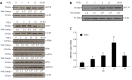
Results: Pretreatment with 1 μmol/L PGE1 protected against TG-induced apoptosis in both L02 cells and HepG2 cells. PGE1 enhanced the TG-induced expression of C/EBP homologous protein (CHOP), glucose-regulated protein (GRP) 78 and spliced X box-binding protein 1 at 6 h. However, it attenuated their expressions after 24 h. PGE1 alone induced protein and mRNA expressions of GRP78; PGE1 also induced protein expression of DNA damage-inducible gene 34 and inhibited the expressions of phospho-PKR-like ER kinase, phospho-eukaryotic initiation factor 2α and CHOP. Treatment with protein kinase A (PKA)-inhibitor H89 or KT5720 blocked PGE1-induced up-regulation of GRP78. Further, the cytoprotective effect of PGE1 on hepatocytes was not observed after blockade of GRP78 expression by H89 or small interfering RNA specifically targeted against human GRP78.
Conclusion: Our study demonstrates that PGE1 protects against ER stress-induced hepatocyte apoptosis via PKA pathway-dependent induction of GRP78 expression.
Keywords: Apoptosis; Endoplasmic reticulum stress; Glucose-regulated protein 78; Hepatocytes; Protein kinase A; Thapsigargin.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no conflicts interest related to this study.
Figures




References
-
- Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583–594. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

