Predictive factors associated with carcinoid syndrome in patients with gastrointestinal neuroendocrine tumors
- PMID: 29142475
- PMCID: PMC5677203
- DOI: 10.3748/wjg.v23.i40.7283
Predictive factors associated with carcinoid syndrome in patients with gastrointestinal neuroendocrine tumors
Abstract
Aim: To discover unknown factors associated with carcinoid syndrome (CS) with the goal of earlier diagnosis of CS.
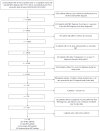
Methods: In this retrospective case-control study using United States administrative claims, patients (≥ 18 years) newly-diagnosed with gastrointestinal neuroendocrine tumors (GI NETs) without CS (controls) were exactly matched to patients with CS (cases) based on NET diagnosis date at a 3-to-1 ratio. Study index date was first CS diagnosis (controls: same distance from NET diagnosis as cases). The most observed conditions, excluding CS-associated symptoms/diagnoses, during the year before index date were assessed. Forward-stepwise logistic regression models were used to derive predictors, and were validation within another claims database.
Results: In the development database, 1004 patients with GI NETs were identified; 251 (25%) had CS and 753 (75%) were controls. In the validation database, 724 patients with GI NETs were identified; 181 (25%) had CS and 543 (75%) were controls. A total of 33 common diagnoses (excluding conditions already known to be associated with CS) in the development database were entered in forward step-wise logistic regression models. In the final, validated logistic regression model, three factors prior to CS diagnosis were found consistently associated with higher risks for CS, including liver disorder [odds ratio (95%CI): 3.38 (2.07-5.51)], enlargement of lymph nodes [2.13 (1.10-4.11)], and abdominal mass [3.79 (1.87-7.69)].
Conclusion: GI NET patients with CS were 2-4 times as likely to have preexisting diagnoses (i.e., liver disorder, enlarged lymph nodes, abdominal mass) than non-CS patients.
Keywords: Carcinoid syndrome; Data mining; Gastrointestinal neuroendocrine tumors; Predictive factors.
Conflict of interest statement
Conflict-of-interest statement: Cai is an employee of Novartis Pharmaceuticals Corporation. Broder, Chang, and Yan are employees of the Partnership for Health Analytic Research, LLC, which received funding from Novartis to conduct the researchdescribed in this manuscript. Metz is an employee of Northwestern University and was paid by Novartis to consult as a subject matter expert. Metz is Chair of the North American Neuroendocrine Tumor Society (NANETS) and also a consultant for Ipsen. Metz has received commercial research grants from Lexicon and Advanced Accelerator Applications (AAA), and is a consultant/on the advisory board for AAA.
Figures
References
-
- National Comprehensive Cancer Network. 2016. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Neuroendocrine Tumors Version 2. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
-
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. - PubMed
-
- Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. - PubMed
-
- Rorstad O. Prognostic indicators for carcinoid neuroendocrine tumors of the gastrointestinal tract. J Surg Oncol. 2005;89:151–160. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

