Expression of CCL2 and its receptor in activation and migration of microglia and monocytes induced by photoreceptor apoptosis
- PMID: 29142497
- PMCID: PMC5669614
Expression of CCL2 and its receptor in activation and migration of microglia and monocytes induced by photoreceptor apoptosis
Abstract
Purpose: To explore the effect of the CCL2 and CCR2 system on the activation and migration of microglia and monocytes in light-induced photoreceptor apoptosis.
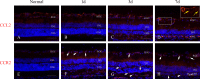
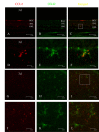
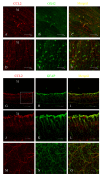
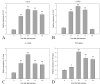
Methods: At 1 day, 3 days, 7 days, and 14 days after light exposure, OX42 and ED1 immunostaining were used to label the activation and migration of microglia and monocytes. Double immunostaining of CCL2 with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), OX42, or glial fibrillary acidic protein (GFAP) was applied to explore the relationships among CCL2, apoptotic photoreceptors, activated microglia and monocytes, and macroglial cells (Müller cells and astrocytes). Real-time PCR was used to evaluate the mRNA levels of retinal CCL2 and CCR2 and the proinflammatory factors interleukin (IL)-1 beta and tumor necrosis factor (TNF)-alpha.
Results: Real-time PCR analyses showed that CCL2 and CCR2 expression gradually increased after light exposure and peaked at 3 days, coinciding with the infiltration of OX42-positive cells and the expression of IL-1 beta and TNF-alpha in the outer retina. Double immunostaining of CCL2 with TUNEL revealed that CCL2 was expressed robustly in about 30% of the apoptotic photoreceptors at the early stage. As degeneration progressed, immunostaining of CCL2 with OX42 showed that activated and migrated microglia and monocytes expressed CCL2. At the late stage, Müller cells became the main source of CCL2, which was illustrated by CCL2 immunostaining with GFAP.
Conclusions: Light exposure led to apoptosis of photoreceptors, which expressed CCL2, accelerating an inflammation-mediated cascade by activating and attracting microglia and monocytes and promoting their secretion of CCL2 in the injured position.
Figures




References
-
- Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med. 2006;38:333–47. - PubMed
-
- Hughes EH, Schlichtenbrede FC, Murphy CC, Sarra GM, Luthert PJ, Ali RR, Dick AD. Generation of activated sialoadhesin-positive microglia during retinal degeneration. Invest Ophthalmol Vis Sci. 2003;44:2229–34. - PubMed
-
- Sennlaub F, Auvynet C, Calippe B, Hu SJ, Dominguez E, Camelo S, Levy O, Guyon E, Saederup N, Charo IF, Rooijen NV, Nandrot E, Bourges JL, Behar-Cohen F, Sahel JA, Guillonneau X, Raoul W, Combadiere C. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol Med. 2013;5:1775–93. - PMC - PubMed
-
- Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T. Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res. 2015;45:30–57. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

