A novel 18F-labelled high affinity agent for PET imaging of the translocator protein
- PMID: 29142713
- PMCID: PMC5667507
- DOI: 10.1039/c5sc01647a
A novel 18F-labelled high affinity agent for PET imaging of the translocator protein
Abstract
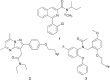
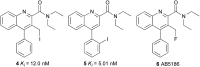
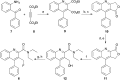
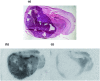
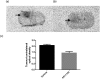
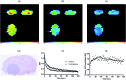
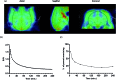
The translocator protein (TSPO) is an important target for imaging focal neuroinflammation in diseases such as brain cancer, stroke and neurodegeneration, but current tracers for non-invasive imaging of TSPO have important limitations. We present the synthesis and evaluation of a novel 3-fluoromethylquinoline-2-carboxamide, AB5186, which was prepared in eight steps using a one-pot two component indium(iii)-catalysed reaction for the rapid and efficient assembly of the 4-phenylquinoline core. Biological assessment and the implementation of a physicochemical study showed AB5186 to have low nanomolar affinity for TSPO, as well as optimal plasma protein binding and membrane permeability properties. Generation of [18F]-AB5186 through 18F incorporation was achieved in good radiochemical yield and subsequent in vitro and ex vivo autoradiography revealed the ability of this compound to bind with specificity to TSPO in mouse glioblastoma xenografts. Initial positron emission tomography imaging of a glioma bearing mouse and a healthy baboon support the potential for [18F]-AB5186 use as a radiotracer for non-invasive TSPO imaging in vivo.
Figures


References
-
- Pimlott S. L., Sutherland A. Chem. Soc. Rev. 2011;40:149–162. - PubMed
-
- Ametamey S. M., Honer M., Schubiger P. A. Chem. Rev. 2008;108:1501–1516. - PubMed
-
- Cai L., Lu S., Pike V. W. Eur. J. Org. Chem. 2008:2853–2873.
-
- Miller P. W., Long N. J., Vilar R., Gee A. D. Angew. Chem., Int. Ed. 2008;47:8998–9033. - PubMed
-
- Scott P. J. Angew. Chem., Int. Ed. 2009;48:6001–6004. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

